Synthesis and in vitro study of surface-modified and anti-EGFR DNA aptamer -conjugated chitosan nanoparticles as a potential targeted drug delivery system
- PMID: 39435057
- PMCID: PMC11491906
- DOI: 10.1016/j.heliyon.2024.e38904
Synthesis and in vitro study of surface-modified and anti-EGFR DNA aptamer -conjugated chitosan nanoparticles as a potential targeted drug delivery system
Abstract
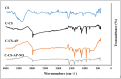
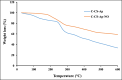
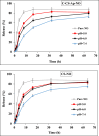
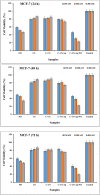
Nowadays, finding effective approaches for cancer therapy is one of the significant issues related to human health all over the world. Hence, in this research, we designed and synthesized a novel targeted DDS based on surface-modified chitosan (CS) for the effective delivery of noscapine (NO). As the surface of CS nanoparticles was firstly modified with carboxyl groups and followed by covalent conjugation of DNA-aptamer (Ap) as targeting and receptor blocker agent. Secondly, NO, as a chemotherapeutic agent, was loaded into prepared nano-complex and synthetics were effectively characterized via various analytical devices, including FT-IR, 1H NMR, DLS, Zeta potential analyzer, TGA, TEM, and SEM to verify quality and quantity of synthetics. Drug loading was obtained about 25 % and sustained drug release was observed for nano-complex at different pHs. Then, the cell viability assay was performed on MCF-7 (as breast cancer cell) and HFF-1 (as normal cell) cell lines to investigate cancer cell inhibition potency of nano-complex. Cell viability of cancer cells was 19.84 ± 1.87 % (for C-CS-Ap-NO) and 75.43 ± 2.64 % (for C-CS-Ap) after 72 h of treatment with 400 nM concentration. These results have been confirming the excellent potency of synthesized novel nano-complex as practical DDS in cancer therapy.
Keywords: Cancer cell inhibition; Chitosan nanoparticles; Controlled noscapine release; DNA-Aptamer; Targeted drug delivery.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Targeted PEGylated Chitosan Nano-complex for Delivery of Sodium Butyrate to Prostate Cancer: An In Vitro Study.Technol Cancer Res Treat. 2023 Jan-Dec;22:15330338231159223. doi: 10.1177/15330338231159223. Technol Cancer Res Treat. 2023. PMID: 36855824 Free PMC article.
-
Synthesis and in vitro study of modified chitosan-polycaprolactam nanocomplex as delivery system.Int J Biol Macromol. 2018 Jul 1;113:1287-1293. doi: 10.1016/j.ijbiomac.2018.02.141. Epub 2018 Feb 23. Int J Biol Macromol. 2018. PMID: 29481956
-
Margetuximab conjugated-PEG-PAMAM G4 nano-complex: a smart nano-device for suppression of breast cancer.Biomed Eng Lett. 2022 Apr 5;12(3):317-329. doi: 10.1007/s13534-022-00225-z. eCollection 2022 Aug. Biomed Eng Lett. 2022. PMID: 35892030 Free PMC article.
-
Targeted Anticancer Drug Delivery Using Chitosan, Carbon Quantum Dots, and Aptamers to Deliver Ganoderic Acid and 5-Fluorouracil.Chem Biodivers. 2023 Sep;20(9):e202300659. doi: 10.1002/cbdv.202300659. Epub 2023 Sep 5. Chem Biodivers. 2023. PMID: 37548485
-
Targeted biocompatible Zn-metal-organic framework nanocomposites for intelligent chemotherapy of breast cancer cells.Sci Rep. 2024 Aug 7;14(1):18311. doi: 10.1038/s41598-024-69457-6. Sci Rep. 2024. PMID: 39112669 Free PMC article.
References
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Ferlay I. Soerjomataram, Dikshit R., Eser S., Mathers C., Rebelo M., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. - PubMed
-
- Yousefi M., Narmani A., Jafari S.M. Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Adv. Colloid Interface Sci. 2020;278 - PubMed
-
- Narmani A., Yavari K., Mohammadnejad J. Imaging, biodistribution and in vitro study of smart 99mTc-PAMAM G4 dendrimer as novel nano-complex. Colloids Surf., B. 2017;159:232–240. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

