Nanomedicine for cancer patient-centered care
- PMID: 39434967
- PMCID: PMC11491554
- DOI: 10.1002/mco2.767
Nanomedicine for cancer patient-centered care
Abstract
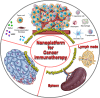
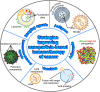
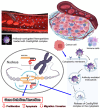
Cancer is a leading cause of morbidity and mortality worldwide, and an increase in incidence is estimated in the next future, due to population aging, which requires the development of highly tolerable and low-toxicity cancer treatment strategies. The use of nanotechnology to tailor treatments according to the genetic and immunophenotypic characteristics of a patient's tumor, and to allow its targeted release, can meet this need, improving the efficacy of treatment and minimizing side effects. Nanomedicine-based approach for the diagnosis and treatment of cancer is a rapidly evolving field. Several nanoformulations are currently in clinical trials, and some have been approved and marketed. However, their large-scale production and use are still hindered by an in-depth debate involving ethics, intellectual property, safety and health concerns, technical issues, and costs. Here, we survey the key approaches, with specific reference to organ-on chip technology, and cutting-edge tools, such as CRISPR/Cas9 genome editing, through which nanosystems can meet the needs for personalized diagnostics and therapy in cancer patients. An update is provided on the nanopharmaceuticals approved and marketed for cancer therapy and those currently undergoing clinical trials. Finally, we discuss the emerging avenues in the field and the challenges to be overcome for the transfer of nano-based precision oncology into clinical daily life.
Keywords: drug delivery systems; nanomedicine; nanotechnology; personalized cancer treatments; precision oncology; targeted therapy.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities.Nanomedicine (Lond). 2019 Jan;14(1):93-126. doi: 10.2217/nnm-2018-0120. Epub 2018 Nov 19. Nanomedicine (Lond). 2019. PMID: 30451076 Free PMC article. Review.
-
Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges.Biomolecules. 2022 Jun 4;12(6):784. doi: 10.3390/biom12060784. Biomolecules. 2022. PMID: 35740909 Free PMC article. Review.
-
Revolutionizing Lung Cancer Treatment: Innovative CRISPR-Cas9 Delivery Strategies.AAPS PharmSciTech. 2024 Jun 6;25(5):129. doi: 10.1208/s12249-024-02834-6. AAPS PharmSciTech. 2024. PMID: 38844700 Review.
-
Biologics, theranostics, and personalized medicine in drug delivery systems.Pharmacol Res. 2024 Mar;201:107086. doi: 10.1016/j.phrs.2024.107086. Epub 2024 Jan 29. Pharmacol Res. 2024. PMID: 38295917 Review.
-
Nanotechnology: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(19):1-43. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2006. PMID: 23074489 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Feynman RP. There's plenty of room at the bottom. Eng Sci. 1960;23(5):22‐36.
-
- ‘Plenty of room’ revisited. Nat Nanotechnol. 2009;4(12):781. - PubMed
-
- Drexler KE. Engines of Creation. The Coming Era of Nanotechnology. New York, NY: Doubleday; 1986.
Publication types
LinkOut - more resources
Full Text Sources
