Diacerein reduces inflammasome activation and SARS-CoV-2 virus replication: a proof-of-concept translational study
- PMID: 39434905
- PMCID: PMC11491754
- DOI: 10.3389/fphar.2024.1402032
Diacerein reduces inflammasome activation and SARS-CoV-2 virus replication: a proof-of-concept translational study
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is linked to high mortality, primarily through an intense inflammatory response. Diacerein has emerged as a potential therapy for COVID-19 due to its potential impact in decreasing the inflammasome activation and coronavirus replication. This study aims to explore diacerein's influence in inhibiting both viral replication and the inflammatory response after SARS-CoV-2 infection.
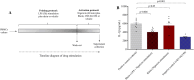
Methods: Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers and infected in vitro with SARS-CoV-2. Additionally, we carried out a pilot randomized, double-blind, placebo-controlled study with 14 participants allocated to diacerein (n = 7) or placebo (n = 7) therapies every 12 h for 10 days. The primary endpoint was change in plasma markers of inflammasome activation (NLRP3, caspase-1, and gasdermin-D).
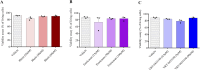
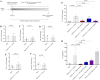
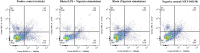
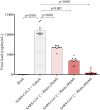
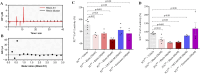
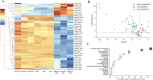
Results: In vitro protocols have shown that rhein, diacerein's primary metabolite, decreased IL-1β secretion caused by SARS-CoV-2 infection in human PBMCs (p < 0.05), and suppressed viral replication when administered either before or after the virus incubation (p < 0.05). This later effect was, at least partially, attributed to its inhibitory effect on 3-chymotrypsin-like protease (SARS-CoV-2 3CLpro) and papain-like protease in the SARS-CoV-2 (SARS-CoV-2 PLpro) virus and in the phosphorylation of proteins related cytoskeleton network (p < 0.05). Diacerein-treated COVID-19 patients presented a smaller area under the curve for NLRP3, caspase-1 and GSDM-D measured on days 2, 5, and 10 after hospitalization compared to those receiving a placebo (p < 0.05).
Conclusion: The indicated mechanisms of action of diacerein/rhein can reduce viral replication and mitigate the inflammatory response related to SARS-CoV-2. These findings are preliminary and require confirmation in clinical trials.
Keywords: COVID-19; clinical trial; diacerein; pre-clinical; rhein.
Copyright © 2024 Carmo, Castillo, Bonilha, Gomes, Barreto, Moura, Davanzo, de Brito Monteiro, Muraro, Fabiano de Souza, Morari, Galdino, Brunetti, Reis-de-Oliveira, Carregari, Nadruz, Martins-de-Souza, Farias, Velloso, Proenca-Modena, Mori, Loh, Bhatt, Yellon, Davidson, De Oliveira, Moraes-Vieira and Sposito.
Conflict of interest statement
DB discloses the following relationships - Advisory Board: Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Stasys; Board of Directors: American Heart Association New York City, Angiowave (stock options), Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock); Consultant: Broadview Ventures, GlaxoSmithKline, Hims, SFJ, Youngene; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hô pitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic, Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical; for ALLAY-HF, funded by Alleviant Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and United States national co-leader, funded by Bayer), WebMD (CME steering committees), (steering committee); Other: Clinical Cardiology (Deputy Editor); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women' s Hospital who assigned to Lexicon; neither I nor Brigham and Women' s Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo. AS discloses the following relationships - Research Funding: Amgen, AstraZeneca, National Council for Scientific and Technological Development (CNPq) and Fundaç and#227; o de Apoio and#224; Pesquisa do Estado de Sã o Paulo (FAPESP). Dr. Pedro Gonç alves de Oliveira is responsible for R&D activities at TRB Pharma Indú stria Quí mica e Farmace utica Ltda, SP, Brazil. TRB Pharma is the owner of the product ARTRODAR® a diacerein-based product for osteoarthritis treatment. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article.
-
Diacerein: A potential multi-target therapeutic drug for COVID-19.Med Hypotheses. 2020 Nov;144:109920. doi: 10.1016/j.mehy.2020.109920. Epub 2020 Jun 1. Med Hypotheses. 2020. PMID: 32534337 Free PMC article.
-
Ivermectin for preventing and treating COVID-19.Cochrane Database Syst Rev. 2022 Jun 21;6(6):CD015017. doi: 10.1002/14651858.CD015017.pub3. Cochrane Database Syst Rev. 2022. PMID: 35726131 Free PMC article. Review.
-
SARS-CoV-2 replication and drug discovery.Mol Cell Probes. 2024 Oct;77:101973. doi: 10.1016/j.mcp.2024.101973. Epub 2024 Jul 24. Mol Cell Probes. 2024. PMID: 39025272 Review.
References
-
- Codo A. C., Davanzo G. G., Monteiro L. B., de Souza G. F., Muraro S. P., Virgilio-da-Silva J. V., et al. (2020). Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 32, 498–499. 10.1016/j.cmet.2020.07.015 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

