The senolytic agent ABT263 ameliorates osteoporosis caused by active vitamin D insufficiency through selective clearance of senescent skeletal cells
- PMID: 39430127
- PMCID: PMC11490840
- DOI: 10.1016/j.jot.2024.08.012
The senolytic agent ABT263 ameliorates osteoporosis caused by active vitamin D insufficiency through selective clearance of senescent skeletal cells
Abstract
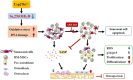
Background/objective: Active vitamin D insufficiency accelerates the development of osteoporosis, with senescent bone cells and the senescence-associated secretory phenotype (SASP) playing crucial roles. This study aimed to investigate whether the senolytic agent ABT263 could correct osteoporosis caused by active vitamin D insufficiency by selectively clearing senescent cells.
Methods: Bone marrow mesenchymal stem cells (BM-MSCs) from young and aged mice were treated with ABT263 in vitro, and 1,25(OH)2D-insufficient (Cyp27b1+/-) mice were administered ABT263 in vivo. Cellular, molecular, imaging, and histopathological analyses were performed to compare treated cells and mice with control groups.
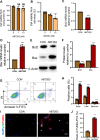
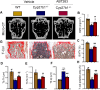
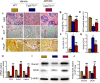
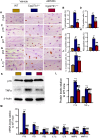
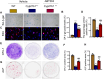
Results: ABT263 induced apoptosis in senescent BM-MSCs by downregulating Bcl2 and upregulating Bax expression. It also induced apoptosis in senescent BM-MSCs from 1,25(OH)2D-insufficient mice. ABT263 administration corrected bone loss caused by 1,25(OH)2D insufficiency by increasing bone density, bone volume, trabecular number, trabecular thickness, and collagen synthesis. It also enhanced osteoblastic bone formation and reduced osteoclastic bone resorption in vivo. ABT263 treatment corrected the impaired osteogenic action of BM-MSCs by promoting their proliferation and osteogenic differentiation. Furthermore, it corrected oxidative stress and DNA damage caused by 1,25(OH)2D insufficiency by increasing SOD-2 and decreasing γ-H2A.X expression. Finally, ABT263 corrected bone cell senescence and SASP caused by 1,25(OH)2D insufficiency by reducing the expression of senescence and SASP-related genes and proteins.
Conclusion: ABT263 can correct osteoporosis caused by active vitamin D insufficiency by selectively clearing senescent skeletal cells, reducing oxidative stress, DNA damage, and SASP, and promoting bone formation while inhibiting bone resorption. These findings provide new insights into the potential therapeutic application of senolytic agents in the treatment of osteoporosis associated with active vitamin D insufficiency.
The translational potential of this article: This study highlights the therapeutic potential of ABT263, a senolytic compound, in treating osteoporosis caused by active vitamin D insufficiency. By selectively eliminating senescent bone cells and their associated SASP, ABT263 intervention demonstrates the ability to restore bone homeostasis, prevent further bone loss, and promote bone formation. These findings contribute to the growing body of research supporting the use of senolytic therapies for the prevention and treatment of age-related bone disorders. The translational potential of this study lies in the development of novel therapeutic strategies targeting cellular senescence to combat osteoporosis, particularly in cases where vitamin D insufficiency is a contributing factor. Further clinical studies are warranted to validate the efficacy and safety of ABT263 and other senolytic agents in the treatment of osteoporosis in humans.
Keywords: Cellular senescence; Osteoporosis; SASP; Senolytics; Vitamin D insufficiency.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
1,25-Dihydroxyvitamin D insufficiency accelerates age-related bone loss by increasing oxidative stress and cell senescence.Am J Transl Res. 2020 Feb 15;12(2):507-518. eCollection 2020. Am J Transl Res. 2020. PMID: 32194899 Free PMC article.
-
1,25-Dihydroxyvitamin D protects against age-related osteoporosis by a novel VDR-Ezh2-p16 signal axis.Aging Cell. 2020 Feb;19(2):e13095. doi: 10.1111/acel.13095. Epub 2019 Dec 26. Aging Cell. 2020. PMID: 31880094 Free PMC article.
-
The Polycomb Protein Bmi1 Plays a Crucial Role in the Prevention of 1,25(OH)2 D Deficiency-Induced Bone Loss.J Bone Miner Res. 2020 Mar;35(3):583-595. doi: 10.1002/jbmr.3921. Epub 2019 Dec 17. J Bone Miner Res. 2020. PMID: 31725940
-
Cellular Senescence in Diabetes Mellitus: Distinct Senotherapeutic Strategies for Adipose Tissue and Pancreatic β Cells.Front Endocrinol (Lausanne). 2022 Mar 31;13:869414. doi: 10.3389/fendo.2022.869414. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432205 Free PMC article. Review.
-
Targeting Cell Senescence for the Treatment of Age-Related Bone Loss.Curr Osteoporos Rep. 2019 Apr;17(2):70-85. doi: 10.1007/s11914-019-00504-2. Curr Osteoporos Rep. 2019. PMID: 30806947 Review.
References
-
- Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. - PubMed
-
- Lips P., van Schoor N.M. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metabol. 2011;25(4):585–591. - PubMed
-
- Yang R., Zhang J., Li J., Qin R., Chen J., Wang R., et al. Inhibition of Nrf2 degradation alleviates age-related osteoporosis induced by 1,25-Dihydroxyvitamin D deficiency. Free Radic Biol Med. 2022;178:246–261. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

