Total Iridoid Glycosides from Swertia mussotii Franch. Alleviate Cholestasis Induced by α-Naphthyl Isothiocyanate through Activating the Farnesoid X Receptor and Inhibiting Oxidative Stress
- PMID: 39408937
- PMCID: PMC11476520
- DOI: 10.3390/ijms251910607
Total Iridoid Glycosides from Swertia mussotii Franch. Alleviate Cholestasis Induced by α-Naphthyl Isothiocyanate through Activating the Farnesoid X Receptor and Inhibiting Oxidative Stress
Abstract
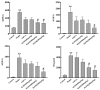
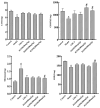
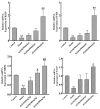
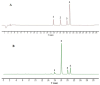
Cholestasis refers to a physiological and pathological process caused by bile acid (BA) overaccumulation inside the circulatory system and liver, leading to systemic and hepatocellular damage. Activating the farnesol X receptor (FXR) to restore BA homeostasis is a promising strategy for treating cholestasis. The objective of this research is to reveal solid evidence for the fact that the total iridoid glycosides from Swertia mussotii Franch. (IGSM) alleviate cholestasis. In this research, the whole plant of S. mussotii was extracted with 70% ethanol and separated by macroporous adsorption resin. A rat cholestasis model was established by the injection of α-naphthyl isothiocyanate (ANIT) at a dose of 75 mg/kg. Biochemical and oxidative stress indicators were determined using commercial assay kits. The mRNA abundance of FXR and target proteins was assessed using RT-qPCR. In addition, the effects of main compounds with FXR were evaluated by molecular docking after IGSM analysis using UPLC. The results indicated that IGSM alleviated ANIT-induced cholestasis through reducing serum ALT, AST, AKP, and TBA levels; increasing the mRNA levels of Fxr, Besp, Ntcp, and Mep2; and reducing oxidative stress. The proportion of iridoid compounds in IGSM exceeded 50%, which may be the active substance basis of IGSM. This study provides a theoretical reference for IGSM in the treatment of cholestasis, and future studies may delve more deeply into the FXR regulatory pathway.
Keywords: FXR; Swertia mussotii Franch.; cholestasis; iridoid glycosides; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

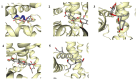
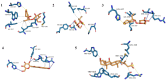
Similar articles
-
Swertiamarin, an active iridoid glycoside from Swertia pseudochinensis H. Hara, protects against alpha-naphthylisothiocyanate-induced cholestasis by activating the farnesoid X receptor and bile acid excretion pathway.J Ethnopharmacol. 2022 Jun 12;291:115164. doi: 10.1016/j.jep.2022.115164. Epub 2022 Mar 9. J Ethnopharmacol. 2022. PMID: 35278607
-
Protective effects of n-Butanol extract and iridoid glycosides of Veronica ciliata Fisch. Against ANIT-induced cholestatic liver injury in mice.J Ethnopharmacol. 2021 Feb 10;266:113432. doi: 10.1016/j.jep.2020.113432. Epub 2020 Oct 1. J Ethnopharmacol. 2021. PMID: 33011367
-
Dolomiaea souliei ethyl acetate extract protected against α-naphthylisothiocyanate-induced acute intrahepatic cholestasis through regulation of farnesoid x receptor-mediated bile acid metabolism.Phytomedicine. 2021 Jul;87:153588. doi: 10.1016/j.phymed.2021.153588. Epub 2021 Jun 3. Phytomedicine. 2021. PMID: 34091148
-
Swertia cincta and its main active ingredients regulate the PPAR-α pathway in anti-cholestatic liver injury.J Ethnopharmacol. 2025 Jan 30;337(Pt 3):118956. doi: 10.1016/j.jep.2024.118956. Epub 2024 Oct 17. J Ethnopharmacol. 2025. PMID: 39423946
-
Targeting FXR in cholestasis: hype or hope.Expert Opin Ther Targets. 2014 Dec;18(12):1449-59. doi: 10.1517/14728222.2014.956087. Epub 2014 Sep 9. Expert Opin Ther Targets. 2014. PMID: 25200104 Review.
References
-
- Hegade V.S., Jones D.E.J. Complications of cholestasis. Medicine. 2019;47:818–821. doi: 10.1016/j.mpmed.2019.09.009. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

