Effect of Isoflavone on Muscle Atrophy in Ovariectomized Mice
- PMID: 39408262
- PMCID: PMC11478932
- DOI: 10.3390/nu16193295
Effect of Isoflavone on Muscle Atrophy in Ovariectomized Mice
Abstract
Background: Sarcopenia, characterized by muscle mass decline due to aging or other causes, is exacerbated by decreased estrogen levels after menopause in women. Isoflavones, a class of flavonoids acting on estrogen receptors, may have beneficial effects on metabolic disorders. We examined these effects in ovariectomized mice fed a high-fat, high-sucrose diet (HFHSD).
Methods: At 7 weeks old, female C57BL6/J mice (18-20 g, n = 12) underwent bilateral ovariectomy (OVX), and were then fed a high-fat, high-sucrose diet starting at 8 weeks of age. Half of the mice received isoflavone water (0.1%). Metabolic analyses, including glucose and insulin tolerance tests, were conducted. Muscle analysis involved grip strength assays, next-generation sequencing, quantitative RT-PCR, and western blotting of skeletal muscle after euthanizing the mice at 14 weeks old. Additionally, 16S rRNA gene sequence analysis of the gut microbiota was performed.
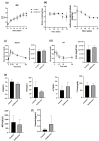
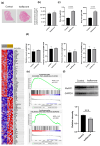
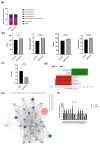
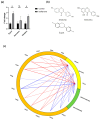
Results: The results demonstrated that isoflavone administration did not affect body weight, glucose tolerance, or lipid metabolism. In contrast, isoflavone-treated mice had higher grip strength. Gene expression analysis of the soleus muscle revealed decreased Trim63 expression, and western blotting showed inactivation of muscle-specific RING finger protein 1 in isoflavone-treated mice. Gut microbiota analysis indicated higher Bacteroidetes and lower Firmicutes abundance in the isoflavone group, along with increased microbiota diversity. Gene sets related to TNF-α signaling via NF-κB and unfolded protein response were negatively associated with isoflavones.
Conclusions: Isoflavone intake alters gut microbiota and increases muscle strength, suggesting a potential role in improving sarcopenia in menopausal women.
Keywords: estrogen; gut microbiota; isoflavone; menopause; sarcopenia.
Conflict of interest statement
Nakajima, H. received individual compensation from Kowa Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd. Nakanishi, N. received individual compensation from Kowa Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Nippon Boehringer Ingelheim Co., Ltd., and TERUMO CORPORATION. Hamaguchi, M. received grants from AstraZeneca K.K., Ono Pharma Co., Ltd., and Kowa Pharmaceutical Co., Ltd.; and also received individual compensation from AstraZeneca K.K., Ono Pharma Co., Ltd., Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corp., Sanofi K.K., and Kowa Pharmaceutical Co., Ltd. outside of the submitted work. Fukui, M. received grants from Ono Pharma Co., Ltd., Oishi Kenko Inc., Yamada Bee Farm, Nippon Boehringer Ingelheim Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., Daiichi Sankyo Co., Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd., Astellas Pharma Inc., MSD K.K., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Kowa Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Eli Lilly Japan K.K., Taisho Pharmaceutical Co., Ltd., Terumo Corp., Teijin Pharma Ltd., Nippon Chemiphar Co., Ltd., Abbott Japan Co., Ltd., and Johnson & Johnson K.K. Medical Co., TERUMO CORPORATION, and also received individual compensation from Nippon Boehringer Ingelheim Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corp., Daiichi Sankyo Co., Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd., Astellas Pharma Inc., MSD K.K., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Kowa Pharmaceutical Co., Ltd., Novo Nordisk Pharma Ltd., Ono Pharma Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Eli Lilly Japan K.K., Taisho Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., AstraZeneca K.K., Mochida Pharmaceutical Co., Ltd., Abbott Japan Co., Ltd., Teijin Pharma Ltd., Arkray Inc., Medtronic Japan Co., Ltd., and Nipro Corp., TERUMO CORPORATION, outside of the submitted work. Sasano, R. was employed by the company AiSTI SCIENCE Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Daidzein Inhibits Muscle Atrophy by Suppressing Inflammatory Cytokine- and Muscle Atrophy-Related Gene Expression.Nutrients. 2024 Sep 13;16(18):3084. doi: 10.3390/nu16183084. Nutrients. 2024. PMID: 39339684 Free PMC article.
-
Modulation of the Gut Microbiota Structure with Probiotics and Isoflavone Alleviates Metabolic Disorder in Ovariectomized Mice.Nutrients. 2021 May 25;13(6):1793. doi: 10.3390/nu13061793. Nutrients. 2021. PMID: 34070274 Free PMC article.
-
The gut microbiota during the progression of atherosclerosis in the perimenopausal period shows specific compositional changes and significant correlations with circulating lipid metabolites.Gut Microbes. 2021 Jan-Dec;13(1):1-27. doi: 10.1080/19490976.2021.1880220. Gut Microbes. 2021. PMID: 33691599 Free PMC article.
-
Long-term silk peptide intake promotes skeletal muscle mass, reduces inflammation, and modulates gut microbiota in middle-aged female rats.Biomed Pharmacother. 2021 May;137:111415. doi: 10.1016/j.biopha.2021.111415. Epub 2021 Feb 23. Biomed Pharmacother. 2021. PMID: 33761619
-
Gut microbiota and metabolic marker alteration following dietary isoflavone-photoperiod interaction.Endocrinol Diabetes Metab. 2020 Oct 17;4(1):e00190. doi: 10.1002/edm2.190. eCollection 2021 Jan. Endocrinol Diabetes Metab. 2020. PMID: 33532621 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

