Intestinal Motility Dysfunction in Goto-Kakizaki Rats: Role of the Myenteric Plexus
- PMID: 39404390
- PMCID: PMC11475219
- DOI: 10.3390/cells13191626
Intestinal Motility Dysfunction in Goto-Kakizaki Rats: Role of the Myenteric Plexus
Abstract
Diabetes mellitus is associated with changes in intestinal morphology and the enteric nervous system. We previously reported constipation in Goto-Kakizaki (GK) rats, a non-obese model for type 2 diabetes mellitus.
Aim: The morpho-quantitative analysis of myenteric plexus neurons in the small and large intestines of 120-day-old male GK rats was investigated.
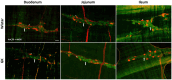
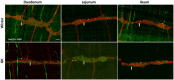
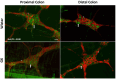
Methods: The diabetes was confirmed by high fasting blood glucose levels. The myenteric plexus was evaluated through wholemount immunofluorescence. The morpho-quantitative analyses included evaluating neuronal density (neurons per ganglion) of the total neuronal population, the cholinergic and nitrergic subpopulations, and enteric glial cells per ganglion. The cell body area of 100 neurons per segment per animal was measured.
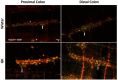
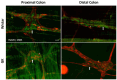
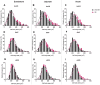
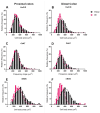
Results: The total neurons and nitrergic subpopulation were unaltered in the GK rats' small and large intestines. The cholinergic subpopulation exhibited decreased density in the three segments of the small intestine and an increased number in the proximal colon of the GK rats. The number of enteric glial cells increased in the ileum of the GK rats, which could indicate enteric gliosis caused by the intestinal inflammatory state. The area of the cell body was increased in the total neuronal population of the jejunum and ileum of the GK rats. Frequency histograms of the cell body area distribution revealed the contribution of cholinergic neurons to larger areas in the jejunum and nitrergic neurons in the ileum.
Conclusion: The constipation previously reported in GK rats might be explained by the decrease in the density of cholinergic neurons in the small intestine of this animal model.
Keywords: Goto-Kakizaki rats; cholinergic neuron; constipation; enteric nervous system; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Small intestine remodeling in male Goto-Kakizaki rats.Physiol Rep. 2021 Feb;9(3):e14755. doi: 10.14814/phy2.14755. Physiol Rep. 2021. PMID: 33580916 Free PMC article.
-
Effects of diabetes mellitus on myenteric neuronal density and sodium channel expression in the rat ileum.Brain Res. 2019 Apr 1;1708:1-9. doi: 10.1016/j.brainres.2018.11.041. Epub 2018 Nov 28. Brain Res. 2019. PMID: 30500400
-
Intestinal and neuronal myenteric adaptations in the small intestine induced by a high-fat diet in mice.BMC Gastroenterol. 2015 Jan 22;15:3. doi: 10.1186/s12876-015-0228-z. BMC Gastroenterol. 2015. PMID: 25609418 Free PMC article.
-
Nitrergic Enteric Neurons in Health and Disease-Focus on Animal Models.Int J Mol Sci. 2019 Apr 24;20(8):2003. doi: 10.3390/ijms20082003. Int J Mol Sci. 2019. PMID: 31022832 Free PMC article. Review.
-
A short review on the features of the non-obese diabetic Goto-Kakizaki rat intestine.Braz J Med Biol Res. 2022 Aug 22;55:e11910. doi: 10.1590/1414-431X2022e11910. eCollection 2022. Braz J Med Biol Res. 2022. PMID: 36000611 Free PMC article. Review.
References
-
- Aroda V.R., Krause-Steinrauf H., Kazemi E.J., Buse J.B., Gulanski B.I., Florez H.J., Ahmann A.J., Loveland A., Kuhn A., Lonier J.Y., et al. Clinical and Metabolic Characterization of Adults With Type 2 Diabetes by Age in the Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) Cohort. Diabetes Care. 2022;45:1512–1521. doi: 10.2337/dc21-2659. - DOI
-
- International Diabetes Federation . IDF Diabetes Atlas. 10th ed. Volume 10th edn IDF Diabetes Atlas; Brussels, Belgium: 2021.
MeSH terms
Grants and funding
- 2018/09868-7/São Paulo State Research Foundation (FAPESP)
- 2019/01942-6/São Paulo State Research Foundation (FAPESP)
- 2022/11249-9/São Paulo State Research Foundation (FAPESP)
- The National Council for Scientific and Technological Development (CNPq)
- Coordination of Superior Level Staff Improvement (CAPES)
LinkOut - more resources
Full Text Sources

