Transcriptional rewiring in CD8+ T cells: implications for CAR-T cell therapy against solid tumours
- PMID: 39399500
- PMCID: PMC11466849
- DOI: 10.3389/fimmu.2024.1412731
Transcriptional rewiring in CD8+ T cells: implications for CAR-T cell therapy against solid tumours
Abstract
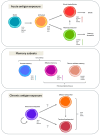
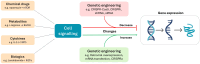
T cells engineered to express chimeric-antigen receptors (CAR-T cells) can effectively control relapsed and refractory haematological malignancies in the clinic. However, the successes of CAR-T cell therapy have not been recapitulated in solid tumours due to a range of barriers such as immunosuppression, poor infiltration, and tumour heterogeneity. Numerous strategies are being developed to overcome these barriers, which include improving culture conditions and manufacturing protocols, implementing novel CAR designs, and novel approaches to engineering the T cell phenotype. In this review, we describe the various emerging strategies to improve CAR T cell therapy for solid tumours. We specifically focus on new strategies to modulate cell function and fate that have precipitated from the growing knowledge of transcriptional circuits driving T cell differentiation, with the ultimate goal of driving more productive anti-tumour T cell immunity. Evidence shows that enrichment of particular phenotypic subsets of T cells in the initial cell product correlates to improved therapeutic responses and clinical outcomes. Furthermore, T cell exhaustion and poor persistence are major factors limiting therapeutic efficacy. The latest preclinical work shows that targeting specific master regulators and transcription factors can overcome these key barriers, resulting in superior T cell therapeutic products. This can be achieved by targeting key transcriptional circuits promoting memory-like phenotypes or sustaining key effector functions within the hostile tumour microenvironment. Additional discussion points include emerging considerations for the field such as (i) targeting permutations of transcription factors, (ii) transient expression systems, (iii) tissue specificity, and (iv) expanding this strategy beyond CAR-T cell therapy and cancer.
Keywords: CAR-T cell; CD8+ T cell; adoptive cell therapy (ACT); cancer; immunotherapy; solid tumour; transcription factor.
Copyright © 2024 Srinivasan, Armitage, Nilsson and Waithman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
NR4A transcription factors limit CAR T cell function in solid tumours.Nature. 2019 Mar;567(7749):530-534. doi: 10.1038/s41586-019-0985-x. Epub 2019 Feb 27. Nature. 2019. PMID: 30814732 Free PMC article.
-
CAR-Ts: new perspectives in cancer therapy.FEBS Lett. 2022 Feb;596(4):403-416. doi: 10.1002/1873-3468.14270. Epub 2022 Jan 12. FEBS Lett. 2022. PMID: 34978080 Review.
-
Chimeric antigen receptor engineered T cells and their application in the immunotherapy of solid tumours.Expert Rev Mol Med. 2022 Jan 28;24:e7. doi: 10.1017/erm.2021.32. Expert Rev Mol Med. 2022. PMID: 35086597 Free PMC article. Review.
-
Engineered CAR-Macrophages as Adoptive Immunotherapies for Solid Tumors.Front Immunol. 2021 Nov 24;12:783305. doi: 10.3389/fimmu.2021.783305. eCollection 2021. Front Immunol. 2021. PMID: 34899748 Free PMC article. Review.
-
Engineering strategies to safely drive CAR T-cells into the future.Front Immunol. 2024 Jun 19;15:1411393. doi: 10.3389/fimmu.2024.1411393. eCollection 2024. Front Immunol. 2024. PMID: 38962002 Free PMC article. Review.
References
-
- D’Angelo SP, Araujo DM, Abdul Razak AR, Agulnik M, Attia S, Blay J-Y, et al. . Afamitresgene autoleucel for advanced synovial sarcoma and myxoid round cell liposarcoma (SPEARHEAD-1): an international, open-label, phase 2 trial. Lancet. (2024) 403:1460–71. doi: 10.1016/S0140-6736(24)00319-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

