The impact of an open-label design on human amniotic membranes vs. silver sulfadiazine dressings for second-degree burns: a randomized controlled clinical trial
- PMID: 39396946
- PMCID: PMC11472429
- DOI: 10.1186/s12893-024-02554-5
The impact of an open-label design on human amniotic membranes vs. silver sulfadiazine dressings for second-degree burns: a randomized controlled clinical trial
Abstract
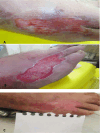
Background: Burn wounds require optimal medical management due to associated psycho-emotional and socioeconomic impacts and severe pain. The use of synthetic and biological dressings improves healing and reduces burn wound complications. The present study aimed to compare the outcomes of using human amniotic membrane (hAM) dressings and conventional silver sulfadiazine (SSDZ) ointment dressings in the management of second-degree burn wounds.
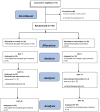
Methods: Fifty patients who participated in this clinical trial were divided into two groups via simple randomization. All the enrolled patients, who had burnt in the last 24 h, had thermal damage mechanisms and were suffering from less than 20% second-degree heat-burn wounds on the skin surface. The target group (n = 25) was treated with hAM, and the control group (n = 25) was treated with SSDZ ointment. The researcher-designed checklist was used to determine the clinical performance in the follow-up assessments on days 7, 14, and 30.
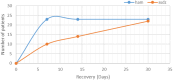
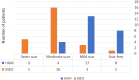
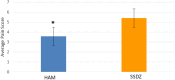
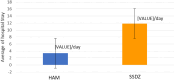
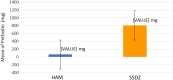
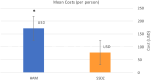
Results: No significant differences were detected in terms of sex, age, or percentage of burn wounds (p > 0.05). Wound epithelialization at days 7, 14, and 30, scar formation, wound pigmentation, pain severity, analgesia requirements, and hospital stay length (on day 30) were significantly lower in the target group (treated with hAM) than in the control group (treated with SSDZ ointment) (p < 0.05). However, treatment costs in the target group ($170) were significantly higher than those in the control group ($71) (p < 0.001).
Conclusion: Despite its higher cost, hAM, as a technology-based therapy dressing, demonstrates superiority over SSDZ ointment in terms of wound healing and pain management.
Keywords: Amniotic membrane; Burns; Clinical trial; Silver sulfadiazine.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Randomized Controlled Trial of Polyhexanide/Betaine Gel Versus Silver Sulfadiazine for Partial-Thickness Burn Treatment.Int J Low Extrem Wounds. 2017 Mar;16(1):45-50. doi: 10.1177/1534734617690949. Epub 2017 Feb 1. Int J Low Extrem Wounds. 2017. PMID: 28682677 Clinical Trial.
-
Antiseptics for burns.Cochrane Database Syst Rev. 2017 Jul 12;7(7):CD011821. doi: 10.1002/14651858.CD011821.pub2. Cochrane Database Syst Rev. 2017. PMID: 28700086 Free PMC article. Review.
-
The effects of Arnebia euchroma ointment on second-degree burn wounds: a randomized clinical trial.J Ethnopharmacol. 2016 Aug 2;189:107-16. doi: 10.1016/j.jep.2016.05.029. Epub 2016 May 13. J Ethnopharmacol. 2016. PMID: 27180881 Clinical Trial.
-
Comparisons of the effects of biological membrane (amnion) and silver sulfadiazine in the management of burn wounds in children.J Burn Care Res. 2011 Mar-Apr;32(2):200-9. doi: 10.1097/BCR.0b013e31820aad94. J Burn Care Res. 2011. PMID: 21258242 Clinical Trial.
-
The role of silver sulphadiazine in the conservative treatment of partial thickness burn wounds: A systematic review.Burns. 2016 Nov;42(7):1377-1386. doi: 10.1016/j.burns.2016.03.029. Epub 2016 Apr 26. Burns. 2016. PMID: 27126813 Review.
References
-
- Opriessnig E, Luze H, Smolle C, Draschl A, Zrim R, Giretzlehner M, Kamolz L-P, Nischwitz SP. Epidemiology of burn injury and the ideal dressing in global burn care – Regional differences explored. Burns. 2023;49(1):1–14. - PubMed
-
- WHO. Burns-WHO | World Health Organization. 2018.
-
- Abraham JP, Plourde BD, Vallez LJ, Nelson-Cheeseman BB, Stark JR, Sparrow EM, Gorman JM. Skin burns. Theory Appl Heat Trans Hum. 2018;2:723–39.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

