Reproducible lung protective effects of a TGFβR1/ALK5 inhibitor in a bleomycin-induced and spirometry-confirmed model of IPF in male mice
- PMID: 39394052
- PMCID: PMC11469938
- DOI: 10.14814/phy2.70077
Reproducible lung protective effects of a TGFβR1/ALK5 inhibitor in a bleomycin-induced and spirometry-confirmed model of IPF in male mice
Abstract
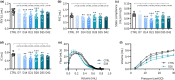
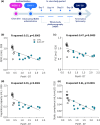
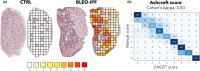
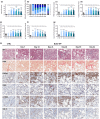
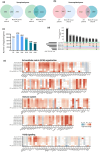
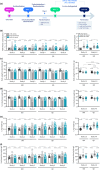
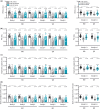
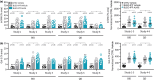
This study comprehensively validated the bleomycin (BLEO) induced mouse model of IPF for utility in preclinical drug discovery. To this end, the model was rigorously evaluated for reproducible phenotype and TGFβ-directed treatment outcomes. Lung disease was profiled longitudinally in male C57BL6/JRJ mice receiving a single intratracheal instillation of BLEO (n = 10-12 per group). A TGFβR1/ALK5 inhibitor (ALK5i) was profiled in six independent studies in BLEO-IPF mice, randomized/stratified to treatment according to baseline body weight and non-invasive whole-body plethysmography. ALK5i (60 mg/kg/day) or vehicle (n = 10-16 per study) was administered orally for 21 days, starting 7 days after intratracheal BLEO installation. BLEO-IPF mice recapitulated functional, histological and biochemical hallmarks of IPF, including declining expiratory/inspiratory capacity and inflammatory and fibrotic lung injury accompanied by markedly elevated TGFβ levels in bronchoalveolar lavage fluid and lung tissue. Pulmonary transcriptome signatures of inflammation and fibrosis in BLEO-IPF mice were comparable to reported data in IPF patients. ALK5i promoted reproducible and robust therapeutic outcomes on lung functional, biochemical and histological endpoints in BLEO-IPF mice. The robust lung fibrotic disease phenotype, along with the consistent and reproducible lung protective effects of ALK5i treatment, makes the spirometry-confirmed BLEO-IPF mouse model highly applicable for profiling novel drug candidates for IPF.
Keywords: ALK5 inhibitor; TGFβ receptor; animal model; bleomycin; deep learning; histopathological scoring; idiopathic pulmonary fibrosis; spirometry; transcriptomics; translatability; whole‐body plethysmography.
© 2024 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
AGP, SHK, JB, DO, SEP, CGS, MWA, MRM, HHH and MFE are employed by Gubra; AGP, DO, CGS, MRM, HHH and MFE are shareholders in Gubra. AMA was employed by Gubra and is presently employed by IQVIA. YN, JB and MRR are employed by Enanta Pharmaceuticals; US is employed by Aarhus University, Aarhus, Denmark. No other potential conflicts of interest relevant to this article were reported.
Figures



Similar articles
-
Preventive and therapeutic effects of thymosin β4 N-terminal fragment Ac-SDKP in the bleomycin model of pulmonary fibrosis.Oncotarget. 2016 Jun 7;7(23):33841-54. doi: 10.18632/oncotarget.8409. Oncotarget. 2016. PMID: 27029074 Free PMC article.
-
The anti-fibrotic effect of inhibition of TGFβ-ALK5 signalling in experimental pulmonary fibrosis in mice is attenuated in the presence of concurrent γ-herpesvirus infection.Dis Model Mech. 2015 Sep;8(9):1129-39. doi: 10.1242/dmm.019984. Epub 2015 Jul 2. Dis Model Mech. 2015. PMID: 26138704 Free PMC article.
-
Human recombinant interferon-alpha2a and interferon-alphaA/D have different effects on bleomycin-induced lung injury.Respiration. 2001;68(2):169-77. doi: 10.1159/000050488. Respiration. 2001. PMID: 11287832
-
Intratracheal bleomycin causes airway remodeling and airflow obstruction in mice.Exp Lung Res. 2012 Apr;38(3):135-46. doi: 10.3109/01902148.2012.658595. Exp Lung Res. 2012. PMID: 22394287 Free PMC article.
-
Allies or enemies? The effect of regulatory T cells and related T lymphocytes on the profibrotic environment in bleomycin-injured lung mouse models.Clin Exp Med. 2023 Aug;23(4):1075-1088. doi: 10.1007/s10238-022-00945-7. Epub 2022 Nov 20. Clin Exp Med. 2023. PMID: 36403186 Free PMC article. Review.
References
-
- Amenta, P. S. , Gil, J. , & Martinez‐Hernandez, A. (1988). Connective tissue of rat lung. II: Ultrastructural localization of collagen types III, IV, and VI. The Journal of Histochemistry and Cytochemistry, 36, 1167–1173. - PubMed
-
- Aran, D. , Looney, A. P. , Liu, L. , Wu, E. , Fong, V. , Hsu, A. , Chak, S. , Naikawadi, R. P. , Wolters, P. J. , Abate, A. R. , Butte, A. J. , & Bhattacharya, M. (2019). Reference‐based analysis of lung single‐cell sequencing reveals a transitional profibrotic macrophage. Nature Immunology, 20, 163–172. - PMC - PubMed
-
- Bedoret, D. , Wallemacq, H. , Marichal, T. , Desmet, C. , Calvo, F. Q. , Henry, E. , Closset, R. , Dewals, B. , Thielen, C. , Gustin, P. , De Leval, L. , Van Rooijen, N. , Le Moine, A. , Vanderplasschen, A. , Cataldo, D. , Drion, P. V. , Moser, M. , Lekeux, P. , & Bureau, F. (2009). Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. The Journal of Clinical Investigation, 119, 3723–3738. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

