Performance evaluation of Eu3+-based CRP/SAA and PCT/IL-6 lateral flow immunoassay kits and their diagnostic value in respiratory tract infections
- PMID: 39391751
- PMCID: PMC11464246
- DOI: 10.1016/j.plabm.2024.e00432
Performance evaluation of Eu3+-based CRP/SAA and PCT/IL-6 lateral flow immunoassay kits and their diagnostic value in respiratory tract infections
Abstract
Objectives: Respiratory infections are among the most common infectious diseases, resulting in significant morbidity and mortality. C-reactive protein (CRP), serum amyloid A (SAA), procalcitonin (PCT), and interleukin-6 (IL-6) are advantageous for diagnosing respiratory tract infections. This study assessed the analytical performance and accuracy of new kits for Eu3+-based CRP/SAA and PCT/IL-6 lateral flow immunoassay and its diagnostic value in respiratory tract infections.
Methods: This study evaluated the detection performance of a test kit using guidelines from the Center for Medical Device Evaluation (CMDE) and the Clinical and Laboratory Standards Institute (CLSI). The test results were compared to those of the commercial kits (CRP: Mindray; SAA: Norman; PCT: Shanghai Upper; IL-6: Wantai BioPharm). A total of 156 patients with respiratory tract infections (53 with bacterial infections (Bac group); 50 with viral infections (Vir group); and 53 with co-infections (Bac + Vir group)) were enrolled, along with 50 healthy controls (HC group). Venous blood samples were collected to measure levels of SAA, PCT, CRP, and IL-6 using both the test and commercial kits. The diagnostic value of these biomarkers was assessed using receiver operating characteristic (ROC) curves.
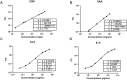
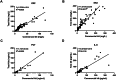
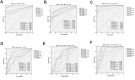
Results: Correlation analysis demonstrated a strong concordance between the test kits and commercial kits (CRP: r = 0.9396, P < 0.0001; SAA: r = 0.8986, P < 0.0001; PCT: r = 0.9594, P < 0.0001; IL-6: r = 0.9009, P < 0.0001). The diagnostic performance of the test kits in identifying bacterial, viral, and co-infections was highly consistent with that of the commercial kit.
Conclusions: The Eu3+-based CRP/SAA and PCT/IL-6 lateral flow immunoassay test kits demonstrated high levels of consistency with commercial kits in terms of quantitative outcomes and diagnostic performance for respiratory tract infections.
Keywords: Detection performance; Diagnostic performance; Lateral flow immunoassay; Respiratory tract infections.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
The Clinical Diagnostic Values of SAA, PCT, CRP, and IL-6 in Children with Bacterial, Viral, or Co-Infections.Int J Gen Med. 2021 Oct 24;14:7107-7113. doi: 10.2147/IJGM.S327958. eCollection 2021. Int J Gen Med. 2021. PMID: 34729020 Free PMC article.
-
Value of combined detection of serum amyloid A, C-reactive protein and procalcitonin in differential diagnosis of respiratory tract infection in children of China.Ann Med. 2022 Dec;54(1):1732-1737. doi: 10.1080/07853890.2022.2064542. Ann Med. 2022. PMID: 35775463 Free PMC article.
-
Clinical diagnostic value of IL-14, 1L-16 and SAA in periodontitis.Clin Oral Investig. 2023 Nov;27(11):6627-6635. doi: 10.1007/s00784-023-05269-8. Epub 2023 Sep 16. Clin Oral Investig. 2023. PMID: 37714977
-
[Diagnosis of bacterial and viral infection by HNL, SAA, PCT and CRP combined test].Zhonghua Yu Fang Yi Xue Za Zhi. 2023 Dec 6;57(12):2153-2158. doi: 10.3760/cma.j.cn112150-20230830-00144. Zhonghua Yu Fang Yi Xue Za Zhi. 2023. PMID: 38186170 Chinese.
-
Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: a systematic review and meta-analysis.Diagn Microbiol Infect Dis. 2014 Sep;80(1):72-8. doi: 10.1016/j.diagmicrobio.2014.03.029. Epub 2014 May 21. Diagn Microbiol Infect Dis. 2014. PMID: 24974271 Review.
References
-
- Blasi F., Torres A. Respiratory infections management: still a challenge. Pulm. Pharmacol. Therapeut. 2015;32:117–118. - PubMed
-
- Zhang Y., Zhang J., Sheng H., Li H., Wang R. Acute phase reactant serum amyloid A in inflammation and other diseases. Adv. Clin. Chem. 2019;90:25–80. - PubMed
-
- Prendki V., Malezieux-Picard A., Azurmendi L., Sanchez J.C., Vuilleumier N., Carballo S., Roux X., Reny J.L., Zekry D., Stirnemann J., et al. Accuracy of C-reactive protein, procalcitonin, serum amyloid A and neopterin for low-dose CT-scan confirmed pneumonia in elderly patients: a prospective cohort study. PLoS One. 2020;15(9) - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

