The role of extracellular vesicles in the pathogenesis of gynecological cancer
- PMID: 39391238
- PMCID: PMC11464257
- DOI: 10.3389/fonc.2024.1477610
The role of extracellular vesicles in the pathogenesis of gynecological cancer
Abstract
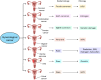
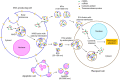
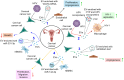
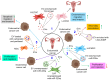
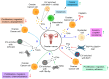
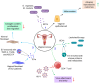
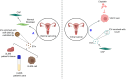
Gynecological cancer, the most common form of cancers in women worldwide, initiates in the reproductive organs of females. More often, the common treatment measures, i.e. surgery, radiation, and medical oncology are found to be unsuccessful in the treatment of gynecological tumors. Emerging evidence indicates that extracellular vesicles (EVs) play a significant role in the pathogenesis of gynecological cancers by distinct mechanisms. The present review highlights how EVs contribute to the progression of different types of gynecological cancers such as cervical cancer, endometrial cancer, ovarian cancer, vaginal cancer, uterine sarcoma, gestational trophoblastic disease (GTD), and vulvar cancer. The primary focus is to understand how EVs' cargo alters the phenotypic response of the recipient cells, thereby contributing to the progression of the disease, thus can be considered as a prognostic and diagnostic biomarker. A brief discussion on the role of EVs in the diagnosis and prognosis of different gynecological cancer types is also highlighted. Targeting the biogenesis of the EVs, their inside cargo, and EVs uptake by the recipient cells could be a potential therapeutic approach in the treatment of gynecological cancer beside conventional therapeutic means.
Keywords: biomarkers; cancer progression; extracellular vesicles; gynecological cancer; therapeutic potential.
Copyright © 2024 Chatterjee, Gupta, Mukherjee, Parashar, Kumar, Maitra and Das.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
[Clinical analysis of 23 cases with simultaneous double primary gynecological malignant tumors].Zhonghua Fu Chan Ke Za Zhi. 2022 May 25;57(5):352-360. doi: 10.3760/cma.j.cn112141-20220214-00086. Zhonghua Fu Chan Ke Za Zhi. 2022. PMID: 35658326 Chinese.
-
Extracellular vesicles and their content in the context of polycystic ovarian syndrome and endometriosis: a review.J Ovarian Res. 2024 Aug 5;17(1):160. doi: 10.1186/s13048-024-01480-7. J Ovarian Res. 2024. PMID: 39103867 Free PMC article. Review.
-
The Emerging Roles of Extracellular Vesicles in Ovarian Cancer.Curr Drug Metab. 2021;22(2):139-149. doi: 10.2174/1389200221666201110155721. Curr Drug Metab. 2021. PMID: 33172376 Review.
-
Extracellular vesicles (EVs)' journey in recipient cells: from recognition to cargo release.J Zhejiang Univ Sci B. 2024 Aug 15;25(8):633-655. doi: 10.1631/jzus.B2300566. J Zhejiang Univ Sci B. 2024. PMID: 39155778 Free PMC article. Review.
-
Extracellular Vesicles and Their Role in Urologic Malignancies.Eur Urol. 2016 Aug;70(2):323-31. doi: 10.1016/j.eururo.2016.02.046. Epub 2016 Feb 28. Eur Urol. 2016. PMID: 26924769 Review.
References
-
- Pahwa S, Kaur A. Statistical analysis of gynecological cancer. Int J Reproduction Contraception Obstetrics Gynecology. (2022) 11:130–6. doi: 10.18203/2320-1770.ijrcog20215089 - DOI
-
- Iyoke CA, Ugwu GO. Burden of gynaecological cancers in developing countries. World J Obstetrics Gynecology. (2013) 2:1–7. doi: 10.5317/wjog.v2.i1.1 - DOI
-
- Sathishkumar K, Sankarapillai J, Mathew A, Nair RA, Gangane N, Khuraijam S, et al. . Survival of patients with cervical cancer in India – findings from 11 population based cancer registries under National Cancer Registry Programme. Lancet Regional Health - Southeast Asia. (2023) 24:100296. doi: 10.1016/j.lansea.2023.100296 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

