Pathways regulating intestinal stem cells and potential therapeutic targets for radiation enteropathy
- PMID: 39388072
- PMCID: PMC11467144
- DOI: 10.1186/s43556-024-00211-0
Pathways regulating intestinal stem cells and potential therapeutic targets for radiation enteropathy
Abstract
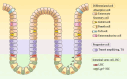
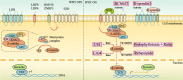
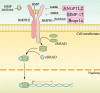
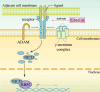
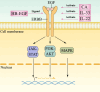
Radiotherapy is a pivotal intervention for cancer patients, significantly impacting their treatment outcomes and survival prospects. Nevertheless, in the course of treating those with abdominal, pelvic, or retroperitoneal malignant tumors, the procedure inadvertently exposes adjacent intestinal tissues to radiation, posing risks of radiation-induced enteropathy upon reaching threshold doses. Stem cells within the intestinal crypts, through their controlled proliferation and differentiation, support the critical functions of the intestinal epithelium, ensuring efficient nutrient absorption while upholding its protective barrier properties. Intestinal stem cells (ISCs) regulation is intricately orchestrated by diverse signaling pathways, among which are the WNT, BMP, NOTCH, EGF, Hippo, Hedgehog and NF-κB, each contributing to the complex control of these cells' behavior. Complementing these pathways are additional regulators such as nutrient metabolic states, and the intestinal microbiota, all of which contribute to the fine-tuning of ISCs behavior in the intestinal crypts. It is the harmonious interplay among these signaling cascades and modulating elements that preserves the homeostasis of intestinal epithelial cells (IECs), thereby ensuring the gut's overall health and function. This review delves into the molecular underpinnings of how stem cells respond in the context of radiation enteropathy, aiming to illuminate potential biological targets for therapeutic intervention. Furthermore, we have compiled a summary of several current treatment methodologies. By unraveling these mechanisms and treatment methods, we aspire to furnish a roadmap for the development of novel therapeutics, advancing our capabilities in mitigating radiation-induced intestinal damage.
Keywords: Biological targets; Intestinal stem cells; Radiation enteropathy; Signaling pathway; Treatment methods.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Ghrelin reverts intestinal stem cell loss associated with radiation-induced enteropathy by activating Notch signaling.Phytomedicine. 2021 Jan;81:153424. doi: 10.1016/j.phymed.2020.153424. Epub 2020 Nov 26. Phytomedicine. 2021. PMID: 33278782
-
LIF is essential for ISC function and protects against radiation-induced gastrointestinal syndrome.Cell Death Dis. 2020 Jul 27;11(7):588. doi: 10.1038/s41419-020-02790-6. Cell Death Dis. 2020. PMID: 32719388 Free PMC article.
-
Berberine enhances the function of intestinal stem cells in healthy and radiation-injured mice.Int Immunopharmacol. 2024 Jul 30;136:112278. doi: 10.1016/j.intimp.2024.112278. Epub 2024 May 29. Int Immunopharmacol. 2024. PMID: 38815353
-
Modulation of radiation-induced intestinal injury by radioprotective agents: a cellular and molecular perspectives.Rev Environ Health. 2022 Apr 20;38(2):295-311. doi: 10.1515/reveh-2021-0108. Print 2023 Jun 27. Rev Environ Health. 2022. PMID: 35438851 Review.
-
Radiation-induced intestinal damage: latest molecular and clinical developments.Future Oncol. 2019 Dec;15(35):4105-4118. doi: 10.2217/fon-2019-0416. Epub 2019 Nov 20. Future Oncol. 2019. PMID: 31746639 Review.
References
-
- Moraitis I, Guiu J, Rubert J. Gut microbiota controlling radiation-induced enteritis and intestinal regeneration. Trends Endocrinol Metab. 2023;34(8):489–501. 10.1016/j.tem.2023.05.006. - PubMed
-
- Gandle C, Dhingra S, Agarwal S. Radiation-induced enteritis. Clin Gastroenterol Hepatol. 2020;18(3):A39-a40. 10.1016/j.cgh.2018.11.060. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
