Suppression of the postprandial hyperglycemia in patients with type 2 diabetes by a raw medicinal herb powder is weakened when consumed in ordinary hard gelatin capsules: A randomized crossover clinical trial
- PMID: 39383145
- PMCID: PMC11463819
- DOI: 10.1371/journal.pone.0311501
Suppression of the postprandial hyperglycemia in patients with type 2 diabetes by a raw medicinal herb powder is weakened when consumed in ordinary hard gelatin capsules: A randomized crossover clinical trial
Abstract
Introduction: Contradictory claims about the efficacy of several medicinal plants to promote glycemic control in patients with type 2 diabetes mellitus (T2DM) have been explained by divergences in the administration form and by extrapolation of data obtained from healthy individuals. It is not known whether the antidiabetic effects of traditional herbal medicines are influenced by gelatin capsules. This randomized crossover trial aimed to evaluate the acute effect of a single dose of raw cinnamon consumed orally either dissolved in water as a beverage or as ordinary hard gelatin capsules on postprandial hyperglycemia (>140 mg/dL; >7.8 mmol/L) in T2DM patients elicited by a nutritionally-balanced meal providing 50 g of complex carbohydrates.
Methods: Fasting T2DM patients (n = 19) randomly ingested a standardized meal in five experimental sessions, one alone (Control) and the other after prior intake of 3 or 6 g of crude cinnamon in the form of hard gelatin capsules or powder dissolved in water. Blood glucose was measured at fasting and at 0.25, 0.5, 0.75, 1, 1.5 and 2 hours postprandially. After each breakfast, its palatability scores for visual appeal, smell and pleasantness of taste were assessed, as well as the taste intensity sweetness, saltiness, bitterness, sourness and creaminess.
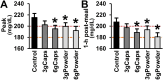
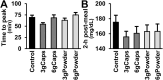
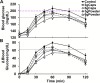
Results: The intake of raw cinnamon dissolved in water, independently of the dose, decreased the meal-induced large glucose spike (peak-rise of +87 mg/dL and Δ1-hour glycemia of +79 mg/dL) and the hyperglycemic blood glucose peak. When cinnamon was taken as capsules, these anti-hyperglycemic effects were lost or significantly diminished. Raw cinnamon intake did not change time-to-peak or the 2-h post-meal glycaemia, but flattened the glycemic curve (lower iAUC) without changing the shape that is typical of T2DM patients.
Conclusions: This cinnamon's antihyperglycemic action confirms its acarbose-like property to inhibit the activities of the carbohydrate-digesting enzymes α-amylases/α-glucosidases, which is in accordance with its exceptionally high content of raw insoluble fiber. The efficacy of using raw cinnamon as a diabetes treatment strategy seems to require its intake at a specific time before/concomitantly the main hyperglycemic daily meals. Trial registration: Registro Brasileiro de Ensaios Clínicos (ReBEC), number RBR-98tx28b.
Copyright: © 2024 Moreira et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
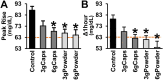
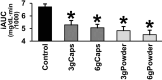
Similar articles
-
Postprandial hyperglycemia in patients with type 2 diabetes is reduced by raw insoluble fiber: A randomized trial.Nutr Metab Cardiovasc Dis. 2024 Dec;34(12):2673-2679. doi: 10.1016/j.numecd.2023.09.013. Epub 2023 Dec 10. Nutr Metab Cardiovasc Dis. 2024. PMID: 39306541 Clinical Trial.
-
Acute Flaxseed Intake Reduces Postprandial Glycemia in Subjects with Type 2 Diabetes: A Randomized Crossover Clinical Trial.Nutrients. 2022 Sep 10;14(18):3736. doi: 10.3390/nu14183736. Nutrients. 2022. PMID: 36145115 Free PMC article. Clinical Trial.
-
Acute effect of Ceylon cinnamon extract on postprandial glycemia: alpha-amylase inhibition, starch tolerance test in rats, and randomized crossover clinical trial in healthy volunteers.BMC Complement Altern Med. 2014 Sep 23;14:351. doi: 10.1186/1472-6882-14-351. BMC Complement Altern Med. 2014. PMID: 25249234 Free PMC article. Clinical Trial.
-
Cinnamon intake lowers fasting blood glucose: meta-analysis.J Med Food. 2011 Sep;14(9):884-9. doi: 10.1089/jmf.2010.0180. Epub 2011 Apr 11. J Med Food. 2011. PMID: 21480806 Review.
-
Do Cinnamon Supplements Have a Role in Glycemic Control in Type 2 Diabetes? A Narrative Review.J Acad Nutr Diet. 2016 Nov;116(11):1794-1802. doi: 10.1016/j.jand.2016.07.015. Epub 2016 Sep 8. J Acad Nutr Diet. 2016. PMID: 27618575 Free PMC article. Review.
References
-
- Kwak JS, Park M young, Kwon O. Effect of cassia cinnamon intake on improvement of the glycemic response: An updated meta-analysis: Focus on preparation of dehydrated powder and water extract. J Nutr Heal. 2017;50: 437. doi: 10.4163/jnh.2017.50.5.437 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

