A fur plucking model to study herpes simplex virus reactivation and recurrent disease
- PMID: 39382285
- PMCID: PMC11520289
- DOI: 10.1128/msphere.00783-23
A fur plucking model to study herpes simplex virus reactivation and recurrent disease
Abstract
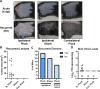
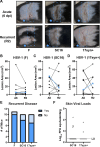
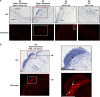
Herpes simplex viruses (HSV-1 and HSV-2) most commonly cause ulcerative epithelial lesions (cold sores and genital herpes). Importantly, HSV establishes life-long persistent (latent) infection in peripheral neurons. Reactivation from latency produces recurrent epithelial lesions, which constitute the greatest burden of HSV disease in people. The mechanisms that regulate latency and reactivation remain incompletely understood, in part due to limitations in the animal models available for studying HSV reactivation. We have developed a simple and tractable model to induce HSV-1 and HSV-2 reactivation from latency to cause recurrent skin disease. We infected C57BL/6 mice with HSV-1 (strains NS, F, SC16, 17syn+) or HSV-2 (strain 333) on flank skin depilated by manual plucking. After at least 35 days post-infection (dpi), we replucked the fur from the infected flank and observed recurrent lesions in the same dermatome as the primary infection. We detected HSV DNA in dermatome skin through 4 days post-replucking and observed viral antigen and reporter signal in skin lesions by histology, consistent with viral replication following reactivation. In addition to C57BL/6 mice, we were able to produce reactivation in Balb/c and SKH-1 mice. We found that shaving the ipsilateral flank or plucking the contralateral flank did not induce recurrent skin lesions, suggesting that fur plucking is a specific stimulus that induces HSV reactivation. Furthermore, we were able to induce multiple rounds of plucking-induced recurrent disease, providing a model to investigate the lifelong nature of HSV infection. This new model provides a tractable system for studying pathogenic mechanisms of and therapeutic interventions against HSV reactivation and recurrent disease.
Importance: Herpes simplex viruses (HSV-1 and HSV-2) have infected over half of the US adult population to cause a lifelong, persistent infection; however, our understanding of the mechanisms that govern HSV reactivation and recurrent disease is incomplete. This is in part due to limitations in the animal models used to study recurrent disease, which are laborious and inefficient in mice. To address this technical gap, we developed a mouse model in which fur plucking after flank skin infection is sufficient to induce episodes of HSV reactivation and recurrent disease. Our work provides a model for the field to investigate the pathogenic mechanisms of HSV and immune responses during recurrent disease and provides an opportunity to investigate the neurobiology of HSV infection.
Keywords: dorsal root ganglia; herpes simplex virus; latency; reactivation; skin infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Update of
-
A fur plucking model to study herpes simplex virus reactivation and recurrent disease.bioRxiv [Preprint]. 2023 Dec 6:2023.12.06.570385. doi: 10.1101/2023.12.06.570385. bioRxiv. 2023. Update in: mSphere. 2024 Oct 29;9(10):e0078323. doi: 10.1128/msphere.00783-23 PMID: 38106009 Free PMC article. Updated. Preprint.
Similar articles
-
A fur plucking model to study herpes simplex virus reactivation and recurrent disease.bioRxiv [Preprint]. 2023 Dec 6:2023.12.06.570385. doi: 10.1101/2023.12.06.570385. bioRxiv. 2023. Update in: mSphere. 2024 Oct 29;9(10):e0078323. doi: 10.1128/msphere.00783-23 PMID: 38106009 Free PMC article. Updated. Preprint.
-
Herpes Simplex Virus 1 Strains 17syn+ and KOS(M) Differ Greatly in Their Ability To Reactivate from Human Neurons In Vitro.J Virol. 2020 Jul 16;94(15):e00796-20. doi: 10.1128/JVI.00796-20. Print 2020 Jul 16. J Virol. 2020. PMID: 32461310 Free PMC article.
-
An M2 Rather than a TH2 Response Contributes to Better Protection against Latency Reactivation following Ocular Infection of Naive Mice with a Recombinant Herpes Simplex Virus 1 Expressing Murine Interleukin-4.J Virol. 2018 Apr 27;92(10):e00051-18. doi: 10.1128/JVI.00051-18. Print 2018 May 15. J Virol. 2018. PMID: 29491152 Free PMC article.
-
Animal models of herpes simplex virus immunity and pathogenesis.J Neurovirol. 2015 Feb;21(1):8-23. doi: 10.1007/s13365-014-0302-2. Epub 2014 Nov 12. J Neurovirol. 2015. PMID: 25388226 Review.
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

