Antimicrobial activity of novel symmetrical antimicrobial peptides centered on a hydrophilic motif against resistant clinical isolates: in vitro and in vivo analyses
- PMID: 39382284
- PMCID: PMC11537005
- DOI: 10.1128/spectrum.00265-24
Antimicrobial activity of novel symmetrical antimicrobial peptides centered on a hydrophilic motif against resistant clinical isolates: in vitro and in vivo analyses
Abstract
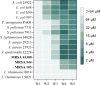
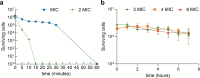
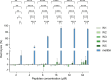
Antibiotic resistance poses a significant public health threat worldwide. The rise in antibiotic resistance and the sharp decline in effective antibiotics necessitate the development of innovative antibacterial agents. Based on the central symmetric structure of glycine-serine-glycine, combined with tryptophan and arginine, we designed a range of antimicrobial peptides (AMPs) that exhibited broad-spectrum antibacterial activity. Notably, AMP W5 demonstrated a rapid and effective sterilization against methicillin-resistant Staphylococcus aureus (MRSA), displaying both a minimum inhibitory concentration and a minimum bactericidal concentration of 8 µM. Mechanistic studies revealed that AMP W5 killed bacterial cells by disrupting the cytoplasmic membrane integrity, triggering leakage of cell contents. AMP W5 also exhibited excellent biocompatibility in both in vitro and in vivo safety evaluations. AMP W5 treatment significantly reduced skin bacterial load in our murine skin infection model. In conclusion, we designed a novel centrosymmetric AMP representing a promising medical alternative to conventional antibiotics for treating MRSA infections.
Importance: Increasing antibiotic resistance and the paucity of effective antibiotics necessitate innovative antibacterial agents. Methicillin-resistant Staphylococcus aureus (MRSA) is a major pathogen causing bacterial infections with high incidence and mortality rates, showing increasing resistance to clinical drugs. Antimicrobial peptides (AMPs) exhibit significant potential as alternatives to traditional antibiotics. This study designed a novel series of AMPs, characterized by a glycine-serine-glycine-centered symmetrical structure, and our results indicated that AMP W5 exhibited a rapid and effective bactericidal effect against MRSA. AMP W5 also demonstrated excellent biocompatibility and a bactericidal mechanism that disrupted membrane integrity, leading to leakage of cellular contents. The notable reduction in skin bacterial load observed in mouse models reinforced the clinical applicability of AMP W5. This study provides a promising solution for addressing the increasing threat of antibiotic-resistant bacteria and heralds new prospects for clinical applications.
Keywords: MRSA; antibiotic resistance; antimicrobial peptides; symmetric sequence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
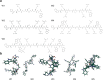


Similar articles
-
Development of Membrane-Targeting Fluorescent 2-Phenyl-1H-phenanthro[9,10-d]imidazole-Antimicrobial Peptide Mimic Conjugates against Methicillin-Resistant Staphylococcus aureus.J Med Chem. 2024 Jun 13;67(11):9302-9317. doi: 10.1021/acs.jmedchem.4c00436. Epub 2024 Mar 16. J Med Chem. 2024. PMID: 38491982
-
Antibacterial efficacy of antimicrobial peptide-functionalized hydrogel particles combined with vancomycin and oxacillin antibiotics.Int J Pharm. 2024 Oct 25;664:124630. doi: 10.1016/j.ijpharm.2024.124630. Epub 2024 Aug 30. Int J Pharm. 2024. PMID: 39216651
-
Cell-Penetrating Antimicrobial Peptides Derived from an Atypical Staphylococcal δ-Toxin.Microbiol Spectr. 2021 Dec 22;9(3):e0158421. doi: 10.1128/spectrum.01584-21. Epub 2021 Dec 22. Microbiol Spectr. 2021. PMID: 34937169 Free PMC article.
-
Advancements in peptide-based antimicrobials: A possible option for emerging drug-resistant infections.Adv Colloid Interface Sci. 2024 Nov;333:103282. doi: 10.1016/j.cis.2024.103282. Epub 2024 Sep 6. Adv Colloid Interface Sci. 2024. PMID: 39276418 Review.
-
Understanding the Dynamics of Human Defensin Antimicrobial Peptides: Pathogen Resistance and Commensal Induction.Appl Biochem Biotechnol. 2024 Oct;196(10):6993-7024. doi: 10.1007/s12010-024-04893-8. Epub 2024 Mar 13. Appl Biochem Biotechnol. 2024. PMID: 38478321 Review.
References
-
- Kourtis AP, Hatfield K, Baggs J, Mu Y, See I, Epson E, Nadle J, Kainer MA, Dumyati G, Petit S, Ray SM, Ham D, Capers C, Ewing H, Coffin N, McDonald LC, Jernigan J, Cardo D, Emerging Infections Program MRSA author group . 2019. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections - United States. MMWR Morb Mortal Wkly Rep 68:214–219. doi:10.15585/mmwr.mm6809e1 - DOI - PMC - PubMed
-
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, Beach M, SENTRY Partcipants Group . 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY antimicrobial surveillance program, 1997-1999. Clin Infect Dis 32 Suppl 2:S114–32. doi:10.1086/320184 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- ZDSYS20220606100803007/Shenzhen Municipal Science and Technology Innovation Council | Shenzhen Key Laboratory Fund
- JCYJ20220818102017035/Shenzhen Municipal Science and Technology Innovation Council | Shenzhen Science and Technology Innovation Program
- JCYJ20190809121801664/Shenzhen Fundamental Research Program
- ChiCTR2100047146/Clinical research 735 Program
LinkOut - more resources
Full Text Sources
Medical

