Oncolytic activity of a coxsackievirus B3 strain in patient-derived cervical squamous cell carcinoma organoids and synergistic effect with paclitaxel
- PMID: 39369233
- PMCID: PMC11452971
- DOI: 10.1186/s12985-024-02502-y
Oncolytic activity of a coxsackievirus B3 strain in patient-derived cervical squamous cell carcinoma organoids and synergistic effect with paclitaxel
Abstract
Background: Cervical squamous cell carcinoma (CSCC) is a prevalent gynecological malignancy worldwide. Current treatments for CSCC can impact fertility and cause long-term complications, underscoring the need for new therapeutic strategies. Oncolytic virotherapy has emerged as a promising option for cancer treatment. Previous research has demonstrated the oncolytic activity of the coxsackievirus B3 strain 2035 A (CVB3/2035A) against various tumor types. This study aims to evaluate the clinical viability of CVB3/2035A for CSCC treatment, focusing on its oncolytic effect in patient-derived CSCC organoids.
Methods: The oncolytic effects of CVB3/2035A were investigated using human CSCC cell lines in vitro and mouse xenograft models in vivo. Preliminary tests for tumor-selectivity were conducted on patient-derived CSCC tissue samples and compared to normal cervical tissues ex vivo. Three patient-derived CSCC organoid lines were developed and treated with CVB3/2035A alone and in combination with paclitaxel. Both cytotoxicity and virus replication were evaluated in vitro.
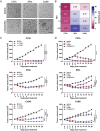
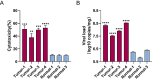
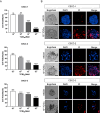
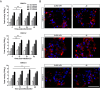
Results: CVB3/2035A exhibited significant cytotoxic effects in human CSCC cell lines and xenograft mouse models. The virus selectively induced oncolysis in patient-derived CSCC tissue samples while sparing normal cervical tissues ex vivo. In patient-derived CSCC organoids, which retained the immunohistological characteristics of the original tumors, CVB3/2035A also demonstrated significant cytotoxic effects and efficient replication, as evidenced by increased viral titers and presence of viral nucleic acids and proteins. Notably, the combination of CVB3/2035A and paclitaxel resulted in enhanced cytotoxicity and viral replication.
Conclusions: CVB3/2035A showed oncolytic activity in CSCC cell lines, xenografts, and patient-derived tissue cultures and organoids. Furthermore, the virus exhibited synergistic anti-tumor effects with paclitaxel against CSCC. These results suggest CVB3/2035A could serve as an alternative or adjunct to current CSCC chemotherapy regimens.
Keywords: Cervical squamous cell carcinoma; Coxsackievirus B3; Oncolytic virus; Organoid; Virotherapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Oncolytic activity of a coxsackievirus B3 strain in human endometrial cancer cell lines.Virol J. 2018 Apr 10;15(1):65. doi: 10.1186/s12985-018-0975-x. Virol J. 2018. PMID: 29631630 Free PMC article.
-
Oncolytic Coxsackievirus B3 Strain PD-H Is Effective Against a Broad Spectrum of Pancreatic Cancer Cell Lines and Induces a Growth Delay in Pancreatic KPC Cell Tumors In Vivo.Int J Mol Sci. 2024 Oct 18;25(20):11224. doi: 10.3390/ijms252011224. Int J Mol Sci. 2024. PMID: 39457005 Free PMC article.
-
Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma.Cancer Res. 2012 May 15;72(10):2609-21. doi: 10.1158/0008-5472.CAN-11-3185. Epub 2012 Mar 29. Cancer Res. 2012. PMID: 22461509
-
Coxsackievirus B3-Its Potential as an Oncolytic Virus.Viruses. 2021 Apr 21;13(5):718. doi: 10.3390/v13050718. Viruses. 2021. PMID: 33919076 Free PMC article. Review.
-
[Oncolytic coxsackievirus therapy as an immunostimulator].Rinsho Ketsueki. 2017;58(8):977-982. doi: 10.11406/rinketsu.58.977. Rinsho Ketsueki. 2017. PMID: 28883283 Review. Japanese.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Lõhmussaar K, Oka R, Espejo Valle-Inclan J, Smits MHH, Wardak H, Korving J, et al. Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell. 2021;28(8):1380–e966. - PubMed
-
- Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393(10167):169–82. - PubMed
-
- Shalhout SZ, Miller DM, Emerick KS, Kaufman HL. Therapy with oncolytic viruses: progress and challenges. Nat Rev Clin Oncol. 2023;20(3):160–77. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

