Cancer cell-specific PD-L1 expression is a predictor of poor outcome in patients with locally advanced oral cavity squamous cell carcinoma
- PMID: 39357980
- PMCID: PMC11448134
- DOI: 10.1136/jitc-2024-009617
Cancer cell-specific PD-L1 expression is a predictor of poor outcome in patients with locally advanced oral cavity squamous cell carcinoma
Abstract
Background: Locally advanced oral cavity squamous cell carcinoma (OCSCC) presents a significant clinical challenge despite being partially responsive to standard treatment modalities. This study investigates the prognostic implications of programmed death-ligand 1 (PD-L1) expression in these tumors, focusing on its association with treatment outcomes and the immune microenvironment.
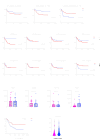
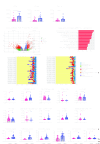
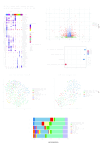
Methods: We assessed tumor-infiltrating lymphocytes (TILs) in 132 patients with OCSCC to evaluate their impact on survival. Multiplex immunohistochemistry staining for CD3, CD68, CD11c, PD-L1, and P40 was used to explore correlations with clinical outcomes in patients with early-stage (n=22) and locally advanced (n=36) OCSCC. These initial findings were validated through differential gene expression analysis, gene set enrichment, and immune cell deconvolution in a The Cancer Genome Atlas cohort of 163 locally advanced OCSCC tumors. Additionally, single-cell RNA sequencing (scRNA-seq) on a smaller cohort (n=10) further characterized the PD-L1hi or PD-L1lo cancer cells in these tumors.
Results: Elevated PD-L1 expression was associated with poor outcomes in patients with locally advanced OCSCC undergoing standard adjuvant therapy, irrespective of "hot" or "cold" classification based on TILs assessment. PD-L1hi tumors exhibited an active immune response phenotype, enriched with M1 macrophages, CD8+ T cells and T regulatory cells in the tumor microenvironment. Notably, the negative impact of PD-L1 expression on outcomes was primarily attributed to its expression by cancer cells, rather than immune cells. Furthermore, scRNA-seq revealed that immune interactions were not essential for PD-L1 upregulation in cancer cells, instead, complex regulatory networks were involved. Additionally, PD-L1lo locally advanced tumors exhibited more complex pathway enrichment and diverse T-cell populations compared with those in the early-stage.
Conclusion: Our findings underscore the prognostic significance of PD-L1 expression in locally advanced OCSCC, and unveil the complex interplay between PD-L1 expression, immune responses, and molecular pathways in the tumor microenvironment. This study provides insights that may inform future therapeutic strategies, including the possibility of tailored immunotherapeutic approaches for patients with PD-L1hi locally advanced OCSCC.
Keywords: Adjuvant; Head and Neck Cancer; Immune Checkpoint Inhibitor; Radiotherapy/Radioimmunotherapy; Tumor Microenvironment.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures


Similar articles
-
Lectin-like Transcript-1 (LLT1) Expression in Oral Squamous Cell Carcinomas: Prognostic Significance and Relationship with the Tumor Immune Microenvironment.Int J Mol Sci. 2024 Apr 13;25(8):4314. doi: 10.3390/ijms25084314. Int J Mol Sci. 2024. PMID: 38673902 Free PMC article.
-
Study of the PD-L1 expression, T-cells density and immunoscore in paired baseline tumor biopsies and surgical specimens in squamous cell carcinoma of the oral cavity.Oral Oncol. 2024 Jul;154:106869. doi: 10.1016/j.oraloncology.2024.106869. Epub 2024 May 30. Oral Oncol. 2024. PMID: 38820890
-
Guanylate binding protein 5 is an immune-related biomarker of oral squamous cell carcinoma: A retrospective prognostic study with bioinformatic analysis.Cancer Med. 2024 Jul;13(13):e7431. doi: 10.1002/cam4.7431. Cancer Med. 2024. PMID: 38978333 Free PMC article.
-
The role of programmed death receptor (PD-)1/PD-ligand (L)1 in periodontitis and cancer.Periodontol 2000. 2024 Oct;96(1):150-169. doi: 10.1111/prd.12548. Epub 2024 Feb 13. Periodontol 2000. 2024. PMID: 38351432 Free PMC article. Review.
-
Assessing PD-L1 Expression in Head and Neck Squamous Cell Carcinoma: Trials and Tribulations.Head Neck Pathol. 2023 Dec;17(4):969-975. doi: 10.1007/s12105-023-01590-6. Epub 2023 Nov 6. Head Neck Pathol. 2023. PMID: 37930471 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
