Herbal medicines for SOD1G93A mice of amyotrophic lateral sclerosis: preclinical evidence and possible immunologic mechanism
- PMID: 39355247
- PMCID: PMC11442286
- DOI: 10.3389/fimmu.2024.1433929
Herbal medicines for SOD1G93A mice of amyotrophic lateral sclerosis: preclinical evidence and possible immunologic mechanism
Abstract
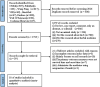
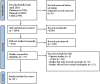
Currently, there is no cure or effective treatment for Amyotrophic Lateral Sclerosis (ALS). The mechanisms underlying ALS remain unclear, with immunological factors potentially playing a significant role. Adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), a systematic review of preclinical studies was conducted, searching seven databases including PubMed, covering literature from the inception of the databases to April 10, 2024. Methodological quality of the included literature was assessed using CAMARADES, while the risk of bias in the included studies was evaluated using SYRCLE's ROB tool. Review Manager 5.4.1 statistical software was used for meta-analysis of the outcomes. The scoping review followed the Joanna Briggs Institute Methodological Guidelines and reporting of this review followed the PRISMA-extension for Scoping Reviews (PRISMA -ScR) checklist to explore the immunological mechanisms of Herbal Medicine (HM) in treating ALS. This systematic review and meta-analysis involved 18 studies with a total of 443 animals. The studies scored between 4 to 8 for methodological quality and 3 to 7 for risk of bias, both summing up to 10.A remarkable effects of HM in ALS mice, including onset time(Standardized Mean Difference(SMD): 1.75, 95% Confidence Interval(CI) (1.14 ~ 2.36), Z = 5.60, P < 0.01), survival time(SMD = 1.42, 95% CI (0.79 ~ 2.04), Z = 4.44, P < 0.01), stride length(SMD=1.90, 95% CI (1.21 to 2.59), Z = 5.39, P < 0.01) and duration time (Mean Difference(MD)=6.79, 95% CI [-0.28, 13.87], Z=1.88, P =0.06), showing HM's certain efficiency in treating ALS mice. The scoping review ultimately included 35 articles for review. HMs may treat ALS through mechanisms such as combating oxidative stress, excitatory amino acid toxicity, and calcium cytotoxicity, understanding and exploring the mechanisms will bring hope to patients. Individual herbs and their formulations within HM address ALS through a variety of immune pathways, including safeguarding the blood-brain barrier, countering neuroinflammation, impeding complement system activation, mitigating natural killer cell toxicity, and regulating T cell-mediated immune pathways. The preclinical evidence supports the utilization of HM as a conventional treatment for ALS mice. Growing evidence indicates that HM may potentially delay neurological degeneration in ALS by activating diverse signaling pathways, especially immune pathways.
Keywords: amyotrophic lateral sclerosis; herbal medicine; mechanism of immunology; meta-analysis; scoping review.
Copyright © 2024 Yang, Wu, Liu and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





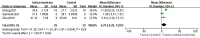
Similar articles
-
Fenretinide Beneficial Effects on Amyotrophic Lateral Sclerosis-associated SOD1G93A Mutant Protein Toxicity: In Vitro and In Vivo Evidences.Neuroscience. 2021 Oct 1;473:1-12. doi: 10.1016/j.neuroscience.2021.07.033. Epub 2021 Aug 5. Neuroscience. 2021. PMID: 34363869
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Effects of cannabinoids in Amyotrophic Lateral Sclerosis (ALS) murine models: a systematic review and meta-analysis.J Neurochem. 2019 Apr;149(2):284-297. doi: 10.1111/jnc.14639. Epub 2019 Jan 18. J Neurochem. 2019. PMID: 30520038
-
Recent progress towards an effective treatment of amyotrophic lateral sclerosis using the SOD1 mouse model in a preclinical setting.Eur J Med Chem. 2016 Oct 4;121:918-925. doi: 10.1016/j.ejmech.2016.02.048. Epub 2016 Feb 23. Eur J Med Chem. 2016. PMID: 27012524 Review.
-
Copper Homeostasis as a Therapeutic Target in Amyotrophic Lateral Sclerosis with SOD1 Mutations.Int J Mol Sci. 2016 Apr 28;17(5):636. doi: 10.3390/ijms17050636. Int J Mol Sci. 2016. PMID: 27136532 Free PMC article. Review.
References
-
- Fang T, Al Khleifat A, Meurgey JH, Jones A, Leigh PN, Bensimon G, et al. . Stage at which riluzole treatment prolongs survival in patients with amyotrophic lateral sclerosis: a retrospective analysis of data from a dose-ranging study. Lancet Neurol. (2018) 17:416–22. doi: 10.1016/S1474-4422(18)30054-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

