Podoplanin expressing macrophages and their involvement in tertiary lymphoid structures in mouse models of Sjögren's disease
- PMID: 39355243
- PMCID: PMC11442383
- DOI: 10.3389/fimmu.2024.1455238
Podoplanin expressing macrophages and their involvement in tertiary lymphoid structures in mouse models of Sjögren's disease
Abstract
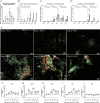
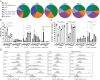
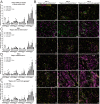
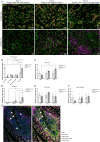
Tertiary lymphoid structures (TLSs) are formed in tissues targeted by chronic inflammation processes, such as infection and autoimmunity. In Sjögren's disease, the organization of immune cells into TLS is an important part of disease progression. Here, we investigated the dynamics of tissue resident macrophages in the induction and expansion of salivary gland TLS. We induced Sjögren's disease by cannulation of the submandibular glands of C57BL/6J mice with LucAdV5. In salivary gland tissues from these mice, we analyzed the different macrophage populations prior to cannulation on day 0 and on day 2, 5, 8, 16 and 23 post-infection using multicolored flow cytometry, mRNA gene analysis, and histological evaluation of tissue specific macrophages. The histological localization of macrophages in the LucAdV5 induced inflamed salivary glands was compared to salivary glands of NZBW/F1 lupus prone mice, a spontaneous mouse model of Sjögren's disease. The evaluation of the dynamics and changes in macrophage phenotype revealed that the podoplanin (PDPN) expressing CX3CR1+ macrophage population was increased in the salivary gland tissue during LucAdV5 induced inflammation. This PDPN+ CX3CR1+ macrophage population was, together with PDPN+CD206+ macrophages, observed to be localized in the parenchyma during the acute inflammation phase as well as surrounding the TLS structure in the later stages of inflammation. This suggests a dual role of tissue resident macrophages, contributing to both proinflammatory and anti-inflammatory processes, as well as their possible interactions with other immune cells within the inflamed tissue. These macrophages may be involved with lymphoid neogenesis, which is associated with disease severity and progression. In conclusion, our study substantiates the involvement of proinflammatory and regulatory macrophages in autoimmune pathology and underlines the possible multifaceted functions of macrophages in lymphoid cell organization.
Keywords: CD206; CX3CR1; Sjögren; autoimmunity; macrophages; mannose receptor; podoplanin; salivary gland.
Copyright © 2024 Hovd, Nayar, Smith, Kanapathippillai, Iannizzotto, Barone, Fenton and Pedersen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
NKp30 Receptor Upregulation in Salivary Glands of Sjögren's Syndrome Characterizes Ectopic Lymphoid Structures and Is Restricted by Rituximab Treatment.Front Immunol. 2021 Sep 14;12:706737. doi: 10.3389/fimmu.2021.706737. eCollection 2021. Front Immunol. 2021. PMID: 34594326 Free PMC article.
-
Myeloid-derived growth factor promotes M2 macrophage polarization and attenuates Sjögren's syndrome via suppression of the CX3CL1/CX3CR1 axis.Front Immunol. 2024 Oct 21;15:1465938. doi: 10.3389/fimmu.2024.1465938. eCollection 2024. Front Immunol. 2024. PMID: 39497829 Free PMC article.
-
Professional antigen presenting cells in minor salivary glands in Sjögren's syndrome: potential contribution to the histopathological diagnosis?Lab Invest. 2000 Dec;80(12):1935-41. doi: 10.1038/labinvest.3780203. Lab Invest. 2000. PMID: 11140705
-
The role of cytokines from salivary gland epithelial cells in the immunopathology of Sjögren's syndrome.Front Immunol. 2024 Sep 13;15:1443455. doi: 10.3389/fimmu.2024.1443455. eCollection 2024. Front Immunol. 2024. PMID: 39346911 Free PMC article. Review.
-
Advances in cellular and molecular pathways of salivary gland damage in Sjögren's syndrome.Front Immunol. 2024 Jul 10;15:1405126. doi: 10.3389/fimmu.2024.1405126. eCollection 2024. Front Immunol. 2024. PMID: 39050857 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

