An Unconventional Case Study of Neoadjuvant Oncolytic Virotherapy for Recurrent Breast Cancer
- PMID: 39339989
- PMCID: PMC11435696
- DOI: 10.3390/vaccines12090958
An Unconventional Case Study of Neoadjuvant Oncolytic Virotherapy for Recurrent Breast Cancer
Abstract
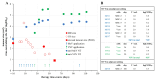
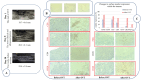
Intratumoural oncolytic virotherapy may have promise as a means to debulk and downstage inoperable tumours in preparation for successful surgery. Here, we describe the unique case of a 50-year-old self-experimenting female virologist with locally recurrent muscle-invasive breast cancer who was able to proceed to simple, non-invasive tumour resection after receiving multiple intratumoural injections of research-grade virus preparations, which first included an Edmonston-Zagreb measles vaccine strain (MeV) and then a vesicular stomatitis virus Indiana strain (VSV), both prepared in her own laboratory. The intratumoural virus therapy was well tolerated. Frequent imaging studies and regular clinical observations documenting size, consistency and mobility of the injected tumour demonstrate that both the MeV- and VSV-containing parts of the protocol contributed to the overall favourable response. Two months after the start of the virus injections, the shrunken tumour was no longer invading the skin or underlying muscle and was surgically excised. The excised tumour showed strong lymphocytic infiltration, with an increase in CD20-positive B cells, CD8-positive T cells and macrophages. PD-L1 expression was detected in contrast to the baseline PD-L1-negative phenotype. The patient completed one-year trastuzumab adjuvant therapy and remains well and recurrence-free 45 months post-surgery. Although an isolated case, it encourages consideration of oncolytic virotherapy as a neoadjuvant treatment modality.
Keywords: Edmonston-Zagreb; breast cancer; measles virus; oncolytic virotherapy; vesicular stomatitis virus.
Conflict of interest statement
BH has become a consultant of Vyriad in 2021. The work described in this paper contains the subject matter of European patent application No. PCT/EP2021/080992 filed on 8 November 2021. The authors declare no conflict of interest.
Figures
Similar articles
-
Oncolytic measles and vesicular stomatitis virotherapy for endometrial cancer.Gynecol Oncol. 2014 Jan;132(1):194-202. doi: 10.1016/j.ygyno.2013.11.010. Epub 2013 Nov 15. Gynecol Oncol. 2014. PMID: 24246772 Free PMC article.
-
Immunovirotherapy with vesicular stomatitis virus and PD-L1 blockade enhances therapeutic outcome in murine acute myeloid leukemia.Blood. 2016 Mar 17;127(11):1449-58. doi: 10.1182/blood-2015-06-652503. Epub 2015 Dec 28. Blood. 2016. PMID: 26712908 Free PMC article.
-
Natural killer T cell immunotherapy combined with IL-15-expressing oncolytic virotherapy and PD-1 blockade mediates pancreatic tumor regression.J Immunother Cancer. 2022 Mar;10(3):e003923. doi: 10.1136/jitc-2021-003923. J Immunother Cancer. 2022. PMID: 35246474 Free PMC article.
-
Measles to the Rescue: A Review of Oncolytic Measles Virus.Viruses. 2016 Oct 22;8(10):294. doi: 10.3390/v8100294. Viruses. 2016. PMID: 27782084 Free PMC article. Review.
-
Potential of vesicular stomatitis virus as an oncolytic therapy for recurrent and drug-resistant ovarian cancer.Chin J Cancer. 2011 Dec;30(12):805-14. doi: 10.5732/cjc.011.10205. Epub 2011 Nov 4. Chin J Cancer. 2011. PMID: 22059911 Free PMC article. Review.
Cited by
-
This scientist treated her own cancer with viruses she grew in the lab.Nature. 2024 Nov;635(8039):529-530. doi: 10.1038/d41586-024-03647-0. Nature. 2024. PMID: 39516696 No abstract available.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

