Interactions of SARS-CoV-2 with Human Target Cells-A Metabolic View
- PMID: 39337465
- PMCID: PMC11432161
- DOI: 10.3390/ijms25189977
Interactions of SARS-CoV-2 with Human Target Cells-A Metabolic View
Abstract
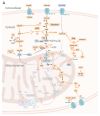
Viruses are obligate intracellular parasites, and they exploit the cellular pathways and resources of their respective host cells to survive and successfully multiply. The strategies of viruses concerning how to take advantage of the metabolic capabilities of host cells for their own replication can vary considerably. The most common metabolic alterations triggered by viruses affect the central carbon metabolism of infected host cells, in particular glycolysis, the pentose phosphate pathway, and the tricarboxylic acid cycle. The upregulation of these processes is aimed to increase the supply of nucleotides, amino acids, and lipids since these metabolic products are crucial for efficient viral proliferation. In detail, however, this manipulation may affect multiple sites and regulatory mechanisms of host-cell metabolism, depending not only on the specific viruses but also on the type of infected host cells. In this review, we report metabolic situations and reprogramming in different human host cells, tissues, and organs that are favorable for acute and persistent SARS-CoV-2 infection. This knowledge may be fundamental for the development of host-directed therapies.
Keywords: COVID-19; SARS-CoV-2; host-directed therapies; long COVID; metabolic reprogramming; persistence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Glycolysis Is an Intrinsic Factor for Optimal Replication of a Norovirus.mBio. 2019 Mar 12;10(2):e02175-18. doi: 10.1128/mBio.02175-18. mBio. 2019. PMID: 30862747 Free PMC article.
-
Metabolic reprogramming as a feast for virus replication.Acta Virol. 2020;64(2):201-215. doi: 10.4149/av_2020_210. Acta Virol. 2020. PMID: 32551788
-
Decoding macrophage immunometabolism in human viral infection.Adv Protein Chem Struct Biol. 2024;140:493-523. doi: 10.1016/bs.apcsb.2023.12.003. Epub 2024 Mar 13. Adv Protein Chem Struct Biol. 2024. PMID: 38762278 Review.
-
Host-Virus Chimeric Events in SARS-CoV-2-Infected Cells Are Infrequent and Artifactual.J Virol. 2021 Jul 12;95(15):e0029421. doi: 10.1128/JVI.00294-21. Epub 2021 Jul 12. J Virol. 2021. PMID: 33980601 Free PMC article.
-
Is Autophagy a Friend or Foe in SARS-CoV-2 Infection?Viruses. 2024 Sep 20;16(9):1491. doi: 10.3390/v16091491. Viruses. 2024. PMID: 39339967 Free PMC article. Review.
References
-
- Modrow S., Falke D., Truyen U., Schätzl H. Viruses with single-stranded, positive-sense RNA genomes. Mol. Virol. 2013:185–349. doi: 10.1007/978-3-642-20718-1_14. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

