Addition of Cryoprotectant DMSO Reduces Damage to Spermatozoa of Yellow Catfish (Pelteobagrus fulvidraco) during Cryopreservation: Ultrastructural Damage, Oxidative Damage and DNA Damage
- PMID: 39335242
- PMCID: PMC11429441
- DOI: 10.3390/ani14182652
Addition of Cryoprotectant DMSO Reduces Damage to Spermatozoa of Yellow Catfish (Pelteobagrus fulvidraco) during Cryopreservation: Ultrastructural Damage, Oxidative Damage and DNA Damage
Abstract
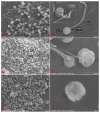
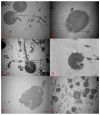
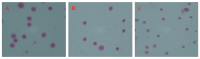
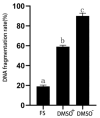
Spermatozoa cryopreservation protocols have been established for yellow catfish (Pelteobagrus fulvidraco), but cryopreservation can still cause cellular damage and affect spermatozoa viability and fertility. Therefore, the aim of this paper was to evaluate the effects of adding or not adding cryoprotectants during low-temperature storage on the ultrastructural damage, oxidative damage, and DNA damage of thawed yellow catfish spermatozoa. The mixed semen of three male yellow catfish was divided into a fresh spermatozoa group, a frozen spermatozoa group (DMSO+) with a cryoprotectant (10% DMSO), and a frozen spermatozoa group without a cryoprotectant (DMSO-). Ultrastructural of the spermatozoa after thawing were observed under an electron microscope and the spermatozoa were assayed for SOD, MDA, and T-AOC enzyme activities, as well as for DNA integrity. In terms of movement parameters, compared with DMSO-, the addition of DMSO has significantly improved sperm motility, curve line velocity (VCL), and straight line velocity (VSL). The ultrastructural results showed that although thawed spermatozoa exhibited increased damage than fresh spermatozoa, 10% DMSO effectively reduced the damage to the plasma membrane, mitochondria, and flagellum of spermatozoa by cryopreservation, and most of the spermatozoa were preserved with intact structure. The results of oxidative damage showed that compared with frozen spermatozoa, 10% DMSO significantly increased the activities of SOD and T-AOC enzymes and clearly reduced the activity of the MDA enzyme. The antioxidant capacity of spermatozoa was improved, lipid peroxidation was reduced, and the oxidative damage caused by cryopreservation was mitigated. The DNA integrity of spermatozoa showed that 10% DMSO clearly reduced the DNA fragmentation rate. In conclusion, 10% DMSO can effectively reduce the ultrastructural damage, oxidative damage, and DNA damage of yellow catfish spermatozoa during cryopreservation; it can also further optimize the cryopreservation protocol for yellow catfish spermatozoa. Meanwhile, it also provides a theoretical basis for the future optimization of the cryopreservation protocols.
Keywords: DMSO; DNA integrity; motility; oxidative damage; spermatozoa cryopreservation; ultrastructure; yellow catfish.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Optimization of conditions for the cryopreservation of yellow catfish (Pelteobagrus fulvidraco) sperm.Cryobiology. 2017 Jun;76:104-110. doi: 10.1016/j.cryobiol.2017.03.009. Epub 2017 Apr 7. Cryobiology. 2017. PMID: 28395972
-
Comparative transcriptome analyses reveal changes of gene expression in fresh and cryopreserved yellow catfish (Pelteobagrus fulvidraco) sperm and the effects of Cryoprotectant Me2SO.Int J Biol Macromol. 2019 Jul 15;133:457-465. doi: 10.1016/j.ijbiomac.2019.04.050. Epub 2019 Apr 16. Int J Biol Macromol. 2019. PMID: 31002905
-
The cryoprotectant used, its concentration, and the equilibration time are critical for the successful cryopreservation of rabbit sperm: DIMETHylacetamide versus dimethylsulfoxide.Theriogenology. 2012 Oct 1;78(6):1381-9. doi: 10.1016/j.theriogenology.2012.06.009. Epub 2012 Aug 13. Theriogenology. 2012. PMID: 22898020
-
Step by step optimization of a sperm cryopreservation protocol for spotted wolffish (Anarhichas minor Olafsen, 1772).Theriogenology. 2020 Jun;149:16-24. doi: 10.1016/j.theriogenology.2020.03.019. Epub 2020 Mar 10. Theriogenology. 2020. PMID: 32229351
-
The characteristics of frozen-thawed rooster sperm using various intracellular cryoprotectants.Poult Sci. 2024 Nov;103(11):104190. doi: 10.1016/j.psj.2024.104190. Epub 2024 Aug 8. Poult Sci. 2024. PMID: 39180781 Free PMC article.
References
-
- Yang Y., Liu D., Wu L., Huang W., Yang S., Xia J., Liu X., Meng Z. Comparative transcriptome analyses reveal changes of gene expression in fresh and cryopreserved yellow catfish (Pelteobagrus fulvidraco) spermatozoa and the effects of Cryocryoprotectant Me2SO. Int. J. Biol. Macromol. 2019;133:457–465. doi: 10.1016/j.ijbiomac.2019.04.050. - DOI - PubMed
-
- Blaxter J.H.S. Spermatozoa storage and cross-fertilization of spring and autumn spawning herring. Nature. 1953;172:1189–1190. doi: 10.1038/1721189b0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

