The Biological Mechanisms and Clinical Roles of RNA-Binding Proteins in Cardiovascular Diseases
- PMID: 39334823
- PMCID: PMC11430443
- DOI: 10.3390/biom14091056
The Biological Mechanisms and Clinical Roles of RNA-Binding Proteins in Cardiovascular Diseases
Abstract
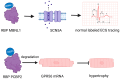
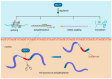
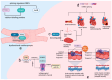
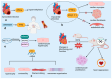
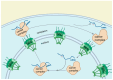
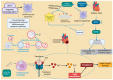
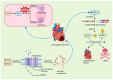
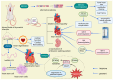
RNA-binding proteins (RBPs) have pivotal roles in cardiovascular biology, influencing various molecular mechanisms underlying cardiovascular diseases (CVDs). This review explores the significant roles of RBPs, focusing on their regulation of RNA alternative splicing, polyadenylation, and RNA editing, and their impact on CVD pathogenesis. For instance, RBPs are crucial in myocardial injury, contributing to disease progression and repair mechanisms. This review systematically analyzes the roles of RBPs in myocardial injury, arrhythmias, myocardial infarction, and heart failure, revealing intricate interactions that influence disease outcomes. Furthermore, the potential of RBPs as therapeutic targets for cardiovascular dysfunction is explored, highlighting the advances in drug development and clinical research. This review also discusses the emerging role of RBPs as biomarkers for cardiovascular diseases, offering insights into their diagnostic and prognostic potential. Despite significant progress, current research faces several limitations, which are critically examined. Finally, this review identifies the major challenges and outlines future research directions to advance the understanding and application of RBPs in cardiovascular medicine.
Keywords: RNA alternative splicing; RNA-binding proteins (RBPs); biomarkers; cardiovascular diseases (CVDs); myocardial injury; therapeutic targets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Genome-wide analysis revealed the dysregulation of RNA binding protein-correlated alternative splicing events in myocardial ischemia reperfusion injury.BMC Med Genomics. 2023 Oct 19;16(1):251. doi: 10.1186/s12920-023-01706-5. BMC Med Genomics. 2023. PMID: 37858115 Free PMC article.
-
Identification of alternative splicing and RNA-binding proteins involved in myocardial ischemia-reperfusion injury.Genome. 2023 Oct 1;66(10):261-268. doi: 10.1139/gen-2022-0102. Epub 2023 Jul 19. Genome. 2023. PMID: 37466303
-
RNA binding proteins in the regulation of heart development.Int J Biochem Cell Biol. 2013 Nov;45(11):2467-78. doi: 10.1016/j.biocel.2013.08.008. Epub 2013 Aug 20. Int J Biochem Cell Biol. 2013. PMID: 23973289 Free PMC article. Review.
-
Emerging Multi-cancer Regulatory Role of ESRP1: Orchestration of Alternative Splicing to Control EMT.Curr Cancer Drug Targets. 2020;20(9):654-665. doi: 10.2174/1568009620666200621153831. Curr Cancer Drug Targets. 2020. PMID: 32564755
-
Cell-specific RNA-binding proteins in human disease.Trends Cardiovasc Med. 2003 Jul;13(5):188-95. doi: 10.1016/s1050-1738(03)00075-6. Trends Cardiovasc Med. 2003. PMID: 12837581 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

