Senescence Rejuvenation through Reduction in Mitochondrial Reactive Oxygen Species Generation by Polygonum cuspidatum Extract: In Vitro Evidence
- PMID: 39334769
- PMCID: PMC11429016
- DOI: 10.3390/antiox13091110
Senescence Rejuvenation through Reduction in Mitochondrial Reactive Oxygen Species Generation by Polygonum cuspidatum Extract: In Vitro Evidence
Abstract
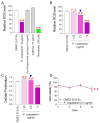
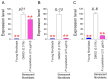
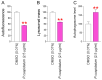
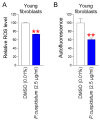
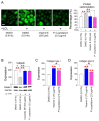
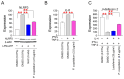
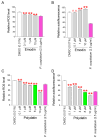
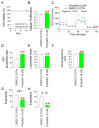
Oxidative stress caused by reactive oxygen species (ROS) is one of the major causes of senescence. Strategies to reduce ROS are known to be important factors in reversing senescence, but effective strategies have not been found. In this study, we screened substances commonly used as cosmetic additives to find substances with antioxidant effects. Polygonum cuspidatum (P. cuspidatum) extract significantly reduced ROS levels in senescent cells. A novel mechanism was discovered in which P. cuspidatum extract reduced ROS, a byproduct of inefficient oxidative phosphorylation (OXPHOS), by increasing OXPHOS efficiency. The reduction in ROS by P. cuspidatum extract restored senescence-associated phenotypes and enhanced skin protection. Then, we identified polydatin as the active ingredient of P. cuspidatum extract that exhibited antioxidant effects. Polydatin, which contains stilbenoid polyphenols that act as singlet oxygen scavengers through redox reactions, increased OXPHOS efficiency and subsequently restored senescence-associated phenotypes. In summary, our data confirmed the effects of P. cuspidatum extract on senescence rejuvenation and skin protection through ROS reduction. This novel finding may be used as a treatment in senescence rejuvenation in clinical and cosmetic fields.
Keywords: Polygonum cuspidatum; oxidative stress; reactive oxygen species (ROS); senescence rejuvenation; skin aging.
Conflict of interest statement
The authors affiliated with Incheon National University (J.H.Y., Y.H.L. and J.T.P.) and Korea University (Y.B.) did not receive any research funding from Hyundai Bioland Co., Ltd. Incheon National University (J.H.Y., Y.H.L. and J.T.P.), Korea University (Y.B.), and Hyundai Bioland Co., Ltd. (E.Y.J., Y.H.K. and S.S.S.) contributed equally to all experiments performed in this study. The authors declare no conflicts of interest. The funders had no role in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the paper.
Figures


Similar articles
-
Polydatin attenuates cadmium-induced oxidative stress via stimulating SOD activity and regulating mitochondrial function in Musca domestica larvae.Chemosphere. 2020 Jun;248:126009. doi: 10.1016/j.chemosphere.2020.126009. Epub 2020 Jan 22. Chemosphere. 2020. PMID: 32000039
-
Unraveling the action mechanism of polygonum cuspidatum by a network pharmacology approach.Am J Transl Res. 2019 Nov 15;11(11):6790-6811. eCollection 2019. Am J Transl Res. 2019. PMID: 31814888 Free PMC article.
-
Investigation of the Active Ingredients and Mechanism of Polygonum cuspidatum in Asthma Based on Network Pharmacology and Experimental Verification.Drug Des Devel Ther. 2021 Mar 9;15:1075-1089. doi: 10.2147/DDDT.S275228. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33727796 Free PMC article.
-
Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: a review.J Ethnopharmacol. 2013 Jul 30;148(3):729-45. doi: 10.1016/j.jep.2013.05.007. Epub 2013 May 22. J Ethnopharmacol. 2013. PMID: 23707210 Review.
-
Advances for pharmacological activities of Polygonum cuspidatum - A review.Pharm Biol. 2023 Dec;61(1):177-188. doi: 10.1080/13880209.2022.2158349. Pharm Biol. 2023. PMID: 36620922 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

