Pharmacological Activation and Transgenic Overexpression of SIRT1 Attenuate Traumatic Optic Neuropathy Induced by Blunt Head Impact
- PMID: 39330985
- PMCID: PMC11437676
- DOI: 10.1167/tvst.13.9.27
Pharmacological Activation and Transgenic Overexpression of SIRT1 Attenuate Traumatic Optic Neuropathy Induced by Blunt Head Impact
Abstract
Purpose: Resveratrol (RSV) is a nutraceutical compound known for its therapeutic potential in neurodegenerative and metabolic diseases. RSV promotes survival signals in retinal ganglion cells (RGCs) through activation of SIRT1, an NAD+-dependent deacetylase. RSV and SIRT1 reduce RGC loss induced by direct optic nerve injury, but effects in indirect models of traumatic optic neuropathy remain unknown and are examined in this study.
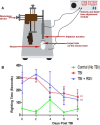
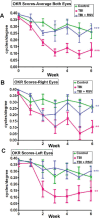
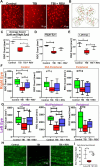
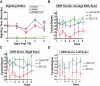
Methods: An electromagnetic stereotaxic impactor device was used to impart five traumatic skull impacts with an inter-concussion interval of 48 hours to wild type (WT) and SIRT1 knock in (KI) C57BL/6J mice overexpressing the SIRT1 gene. A cohort of WT mice also received intranasal administration of RSV (16 mg/kg) throughout the experimental period. Loss of righting reflex (RR), optokinetic response (OKR) scores, and immunolabeled RGC count are determined to assess optic neuropathy in this model of traumatic brain injury (TBI).
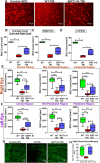
Results: TBI significantly decreases RGC survival and decreases OKR scores compared with control uninjured mice. Either RSV administration in WT mice, or SIRT1 overexpression in SIRT1 KI mice, significantly increases RGC survival and improves OKR scores. RR time increases after the first few impacts in all groups of mice subjected to TBI, demonstrating that RSV and SIRT1 overexpression are able to attenuate optic neuropathy following similar degrees of TBI.
Conclusions: Intranasal RSV is effective in preserving visual function in WT mice following TBI. Constitutive overexpression of SIRT1 recapitulates the neuroprotective effect of RSV.
Translational relevance: Results support future exploration of RSV as a potential therapy for indirect traumatic optic neuropathy.
Conflict of interest statement
Disclosure:
Figures
Similar articles
-
SIRT1 promotes RGC survival and delays loss of function following optic nerve crush.Invest Ophthalmol Vis Sci. 2013 Jul 26;54(7):5097-102. doi: 10.1167/iovs.13-12157. Invest Ophthalmol Vis Sci. 2013. PMID: 23821198 Free PMC article.
-
Rescue of retinal ganglion cells in optic nerve injury using cell-selective AAV mediated delivery of SIRT1.Gene Ther. 2021 May;28(5):256-264. doi: 10.1038/s41434-021-00219-z. Epub 2021 Feb 15. Gene Ther. 2021. PMID: 33589779 Free PMC article.
-
RGC Neuroprotection Following Optic Nerve Trauma Mediated By Intranasal Delivery of Amnion Cell Secretome.Invest Ophthalmol Vis Sci. 2018 May 1;59(6):2470-2477. doi: 10.1167/iovs.18-24096. Invest Ophthalmol Vis Sci. 2018. PMID: 29847652 Free PMC article.
-
Mitochondrial targeted therapy with elamipretide (MTP-131) as an adjunct to tumor necrosis factor inhibition for traumatic optic neuropathy in the acute setting.Exp Eye Res. 2020 Oct;199:108178. doi: 10.1016/j.exer.2020.108178. Epub 2020 Aug 3. Exp Eye Res. 2020. PMID: 32758490 Free PMC article. Review.
-
Psychophysical testing in rodent models of glaucomatous optic neuropathy.Exp Eye Res. 2015 Dec;141:154-63. doi: 10.1016/j.exer.2015.06.025. Epub 2015 Jul 2. Exp Eye Res. 2015. PMID: 26144667 Free PMC article. Review.
References
-
- Levin LA, Beck RW, Joseph MP, Seiff S, Kraker R.. The treatment of traumatic optic neuropathy: the International Optic Nerve Trauma Study. Ophthalmology. 1999; 106: 1268–1277. - PubMed
-
- Bastakis GG, Ktena N, Karagogeos D, Savvaki M.. Models and treatments for traumatic optic neuropathy and demyelinating optic neuritis. Dev Neurobiol. 2019; 79: 819–836. - PubMed
-
- Levin LA. Axonal loss and neuroprotection in optic neuropathies. Can J Ophthalmol. 2007; 42: 403–408. - PubMed
-
- Ropposch T, Steger B, Meco C, et al. .. The effect of steroids in combination with optic nerve decompression surgery in traumatic optic neuropathy. Laryngoscope. 2013; 123: 1082–1086. - PubMed
-
- Yu-Wai-Man P, Griffiths PG.. Steroids for traumatic optic neuropathy. Cochrane Database Syst Rev. 2011; 1: CD006032. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

