Role of circulating T follicular helper subsets following Ty21a immunization and oral challenge with wild type S. Typhi in humans
- PMID: 39328410
- PMCID: PMC11424897
- DOI: 10.3389/fimmu.2024.1384642
Role of circulating T follicular helper subsets following Ty21a immunization and oral challenge with wild type S. Typhi in humans
Abstract
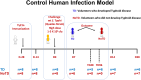
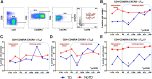
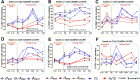
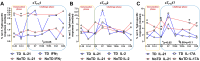
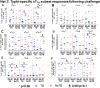
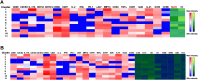
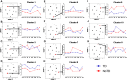
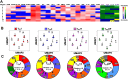
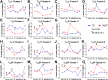
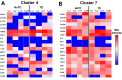
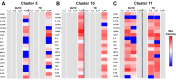
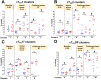
Despite decades of intense research, our understanding of the correlates of protection against Salmonella Typhi (S. Typhi) infection and disease remains incomplete. T follicular helper cells (TFH), an important link between cellular and humoral immunity, play an important role in the development and production of high affinity antibodies. While traditional TFH cells reside in germinal centers, circulating TFH (cTFH) (a memory subset of TFH) are present in blood. We used specimens from a typhoid controlled human infection model whereby participants were immunized with Ty21a live attenuated S. Typhi vaccine and then challenged with virulent S. Typhi. Some participants developed typhoid disease (TD) and some did not (NoTD), which allowed us to assess the association of cTFH subsets in the development and prevention of typhoid disease. Of note, the frequencies of cTFH were higher in NoTD than in TD participants, particularly 7 days after challenge. Furthermore, the frequencies of cTFH2 and cTFH17, but not cTFH1 subsets were higher in NoTD than TD participants. However, we observed that ex-vivo expression of activation and homing markers were higher in TD than in NoTD participants, particularly after challenge. Moreover, cTFH subsets produced higher levels of S. Typhi-specific responses (cytokines/chemokines) in both the immunization and challenge phases. Interestingly, unsupervised analysis revealed unique clusters with distinct signatures for each cTFH subset that may play a role in either the development or prevention of typhoid disease. Importantly, we observed associations between frequencies of defined cTFH subsets and anti-S. Typhi antibodies. Taken together, our results suggest that circulating TFH2 and TFH17 subsets might play an important role in the development or prevention of typhoid disease. The contribution of these clusters was found to be distinct in the immunization and/or challenge phases. These results have important implications for vaccines aimed at inducing long-lived protective T cell and antibody responses.
Keywords: CHIM; S. Typhi; cTfh; circulating follicular helper T cells; typhoid fever.
Copyright © 2024 Booth, Rapaka, McArthur, Fresnay, Darton, Blohmke, Jones, Waddington, Levine, Pollard and Sztein.
Conflict of interest statement
ML: Co-inventor of a live attenuated S. Typhi vaccine strain CVD 909 and a S. Paratyphi A vaccine strain CVD 1902 that have been licensed to Bharat Biotech International BBI, Hyderabad, India for clinical development. ML is also a co-inventor of a Trivalent Salmonella Conjugate vaccine that includes S. Enteritidis, S. Typhimurium conjugates core plus O-polysaccharide covalently linked to FliC flagellin subunits of the homologous serovars in combination with BBI’s Vi conjugate Typbar TCV. AP: is chair of the UK department of Health and Social Cares Joint Committee on vaccination and immunisation. He has research grants on typhoid/paratyphoid vaccines from Serum Institute of India, Medical Research Council, Wellcome Trust and Bill & Melinda gates Foundation. Author MM was employed by company Sanofi. Authors SF and CB were employed by company GlaxsoSmithKline. Author RR is presently employed at Moderna Therapeutics and owns shares/options. Her contributions to this project were made prior to her employment at Moderna. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Specific and cross-reactive immune response to oral Salmonella Typhi Ty21a and parenteral Vi capsular polysaccharide typhoid vaccines administered concomitantly.Vaccine. 2015 Jan 9;33(3):451-8. doi: 10.1016/j.vaccine.2014.11.030. Epub 2014 Nov 26. Vaccine. 2015. PMID: 25433216
-
Using a Human Challenge Model of Infection to Measure Vaccine Efficacy: A Randomised, Controlled Trial Comparing the Typhoid Vaccines M01ZH09 with Placebo and Ty21a.PLoS Negl Trop Dis. 2016 Aug 17;10(8):e0004926. doi: 10.1371/journal.pntd.0004926. eCollection 2016 Aug. PLoS Negl Trop Dis. 2016. PMID: 27533046 Free PMC article. Clinical Trial.
-
Age-Associated Heterogeneity of Ty21a-Induced T Cell Responses to HLA-E Restricted Salmonella Typhi Antigen Presentation.Front Immunol. 2019 Mar 4;10:257. doi: 10.3389/fimmu.2019.00257. eCollection 2019. Front Immunol. 2019. PMID: 30886613 Free PMC article.
-
Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica Serovar Typhi strains used as live oral vaccines in humans.Clin Infect Dis. 2007 Jul 15;45 Suppl 1:S15-9. doi: 10.1086/518140. Clin Infect Dis. 2007. PMID: 17582562 Review.
-
Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi.J Infect Dis. 1984 Sep;150(3):436-49. doi: 10.1093/infdis/150.3.436. J Infect Dis. 1984. PMID: 6207249 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

