Parthenolide ameliorates 3-nitropropionic acid-induced Huntington's disease-like aberrations via modulating NLRP3 inflammasome, reducing microglial activation and inducing astrocyte shifting
- PMID: 39327568
- PMCID: PMC11425901
- DOI: 10.1186/s10020-024-00917-5
Parthenolide ameliorates 3-nitropropionic acid-induced Huntington's disease-like aberrations via modulating NLRP3 inflammasome, reducing microglial activation and inducing astrocyte shifting
Abstract
Background: Huntington's disease (HD) is a progressive neurodegenerative disease that causes motor, cognitive, and psychiatric abnormalities, with no satisfying disease-modifying therapy so far. 3-nitropropionic acid (3NP) induces behavioural deficits, together with biochemical and histological alterations in animals' striata that mimic HD. The role of nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome in HD pathogenesis remains largely uncharacterized. Parthenolide (PTL), a naturally occurring nuclear factor kappa B (NF-κB) inhibitor, is also known to inhibit NLRP3 inflammasome. Whether PTL is beneficial in HD has not been established yet.
Aim: This study evaluated the possible neuroprotective effects of PTL against 3NP-induced behavioural abnormalities, striatal biochemical derangements, and histological aberrations.
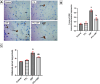
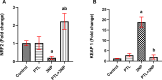
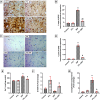
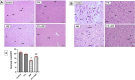
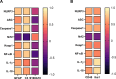
Methods: Male Wistar rats received PTL (0.5 mg/kg/day, i.p) for 3 weeks and 3NP (10 mg/kg/day, i.p) was administered alongside for the latter 2 weeks to induce HD. Finally, animals were subjected to open-field, Morris water maze and rotarod tests. Rat striata were examined histologically, striatal protein expression levels of glial fibrillary acidic protein (GFAP), cluster of differentiation 45 (CD45) and neuron-specific enolase (NSE) were evaluated immunohistochemically, while those of interleukin (IL)-1β, IL-18, ionized calcium-binding adapter molecule-1 (Iba1) and glutamate were determined by ELISA. Striatal nuclear factor erythroid 2-related factor 2 (Nrf2), Kelch-like ECH-associated protein (Keap1), NF-κB, NLRP3, apoptosis-associated speck-like protein containing a CARD (ASC), caspase-1, S100 calcium-binding protein A10 (S100A10) and complement-3 (C3) were assessed by gene expression analysis.
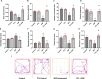
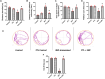
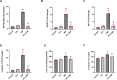
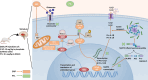
Results: PTL improved motor, locomotor, cognitive and anxiety-like behaviours, restored neuronal integrity, upregulated Nrf2, and inhibited NLRP3 inflammasome, NF-κB and microglial activation. Additionally, PTL induced astrocyte shifting towards the neuroprotective A2 phenotype.
Conclusion: PTL exhibits neuroprotection against 3NP-induced HD, that might be ascribed, at least in part, to its modulatory effects on Keap1/Nrf2 and NF-κB/NLRP3 inflammasome signaling.
Keywords: 3-Nitropropionic acid; Huntington’s disease; NF-κB; NLRP3; Neuroinflammation; Parthenolide.
© 2024. The Author(s).
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

Similar articles
-
Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway.Front Immunol. 2019 Jul 2;10:1511. doi: 10.3389/fimmu.2019.01511. eCollection 2019. Front Immunol. 2019. PMID: 31327964 Free PMC article.
-
A selective inhibitor of the NLRP3 inflammasome as a potential therapeutic approach for neuroprotection in a transgenic mouse model of Huntington's disease.J Neuroinflammation. 2022 Feb 26;19(1):56. doi: 10.1186/s12974-022-02419-9. J Neuroinflammation. 2022. PMID: 35219323 Free PMC article.
-
Vildagliptin Attenuates Huntington's Disease through Activation of GLP-1 Receptor/PI3K/Akt/BDNF Pathway in 3-Nitropropionic Acid Rat Model.Neurotherapeutics. 2020 Jan;17(1):252-268. doi: 10.1007/s13311-019-00805-5. Neurotherapeutics. 2020. PMID: 31728850 Free PMC article.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Inflammasome activation and assembly in Huntington's disease.Mol Immunol. 2022 Nov;151:134-142. doi: 10.1016/j.molimm.2022.09.002. Epub 2022 Sep 18. Mol Immunol. 2022. PMID: 36126501 Review.
References
-
- Abdel Rasheed NO, Ibrahim WW. Telmisartan neuroprotective effects in 3-nitropropionic acid Huntington’s disease model in rats: Cross talk between PPAR-γ and PI3K/Akt/GSK-3β pathway. Life Sci. Volume 297. Elsevier Inc.; 2022. p. 120480. January. - PubMed
-
- Abdelfattah MS, Badr SEA, Lotfy SA, Attia GH, Aref AM, Abdel Moneim AE, et al. Rutin and Selenium Co-administration Reverse 3-Nitropropionic Acid-Induced Neurochemical and Molecular impairments in a mouse model of Huntington’s Disease. Neurotox Res Neurotox Res. 2020;37(1):77–92. - PubMed
-
- Abdelmonem M, Ali SO, Al-Mokaddem AK, Ghaiad HR. Ameliorating diabetes-induced testicular dysfunction by modulating PKC/Nrf2/Bcl-2 signaling: Protective role of sulbutiamine. BioFactors. 2024. - PubMed
-
- Ahmed La, Darwish HA, Abdelsalam RM, Amin HAA. Role of Rho Kinase Inhibition in the Protective Effect of Fasudil and Simvastatin Against 3-Nitropropionic Acid-Induced Striatal Neurodegeneration and Mitochondrial Dysfunction in Rats. Mol. Neurobiol. [Internet]. 2016;53(6):3927–38. http://link.springer.com/10.1007/s12035-015-9303-2 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

