Chronic lung inflammation and CK14+ basal cell proliferation induce persistent alveolar-bronchiolization in SARS-CoV-2-infected hamsters
- PMID: 39326207
- PMCID: PMC11470415
- DOI: 10.1016/j.ebiom.2024.105363
Chronic lung inflammation and CK14+ basal cell proliferation induce persistent alveolar-bronchiolization in SARS-CoV-2-infected hamsters
Abstract
Background: Post-acute sequalae of COVID-19 defines a wide range of ongoing symptoms and conditions long after SARS-CoV-2 infection including respiratory diseases. The histopathological changes in the lung and underlying mechanism remain elusive.
Methods: We investigated lung histopathological and transcriptional changes in SARS-CoV-2-infected male hamsters at 7, 14, 42, 84 and 120dpi, and compared with A (H1N1)pdm09 infection.
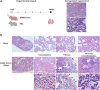
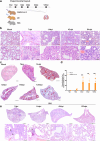
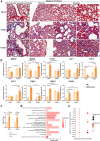
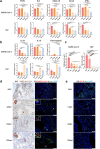
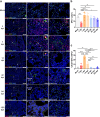
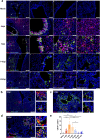
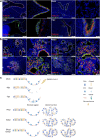
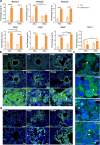
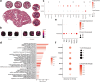
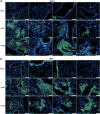
Findings: We demonstrated viral residue, inflammatory and fibrotic changes in lung after SARS-CoV-2 but not H1N1 infection. The most prominent histopathological lesion was multifocal alveolar-bronchiolization observed in every SARS-CoV-2 infected hamster (31/31), from 42dpi to 120dpi. Proliferating (Ki67+) CK14+ basal cells accumulated in alveoli adjacent to bronchioles at 7dpi, where they proliferated and differentiated into SCGB1A+ club cell or Tubulin+ ciliated cells forming alveolar-bronchiolization foci. Molecularly, Notch pathway significantly upregulated with intensive Notch3 and Hes1 protein expression in alveolar-bronchiolization foci at 42 and 120dpi, suggesting Notch signaling involving the persistence of alveolar-bronchiolization. This is further demonstrated by spatial transcriptomic analysis. Intriguingly, significant upregulation of some cell-growth promoting pathways and genes such as Tubb4b, Stxbp4, Grb14 and Mlf1 were spatially overlapping with bronchiolization lesion.
Interpretation: Incomplete resolution of SARS-CoV-2 infection in lung with viral residue, chronic inflammatory and fibrotic damage and alveolar-bronchiolization impaired respiratory function. Aberrant activation of CK14+ basal cells during tissue regeneration led to persistent alveolar-bronchiolization due to sustained Notch signaling. This study advances our understanding of respiratory PASC, sheds light on disease management and highlights the necessity for monitoring disease progression in people with respiratory PASC.
Funding: Funding is listed in the Acknowledgements section.
Keywords: Alveolar-bronchiolization; Basal cell; Hamster; Long COVID-19; PASC; SARS-CoV-2.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Similar articles
-
Quantitative proteomics of hamster lung tissues infected with SARS-CoV-2 reveal host factors having implication in the disease pathogenesis and severity.FASEB J. 2021 Jul;35(7):e21713. doi: 10.1096/fj.202100431R. FASEB J. 2021. PMID: 34105201 Free PMC article.
-
Hamster model for post-COVID-19 alveolar regeneration offers an opportunity to understand post-acute sequelae of SARS-CoV-2.Nat Commun. 2023 Jun 5;14(1):3267. doi: 10.1038/s41467-023-39049-5. Nat Commun. 2023. PMID: 37277327 Free PMC article.
-
Dynamic Changes of the Blood Chemistry in Syrian Hamsters Post-Acute COVID-19.Microbiol Spectr. 2022 Feb 23;10(1):e0236221. doi: 10.1128/spectrum.02362-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196799 Free PMC article.
-
Lesions in the lungs of fatal corona virus disease Covid-19.Ann Anat. 2021 Mar;234:151657. doi: 10.1016/j.aanat.2020.151657. Epub 2020 Dec 3. Ann Anat. 2021. PMID: 33279630 Free PMC article. Review.
-
SARS-CoV-2 Infection and Disease Modelling Using Stem Cell Technology and Organoids.Int J Mol Sci. 2021 Feb 26;22(5):2356. doi: 10.3390/ijms22052356. Int J Mol Sci. 2021. PMID: 33652988 Free PMC article. Review.
References
-
- World Health Organization . WHO Coronavirus (COVID-19) dashboard. 2024. Accessed on May 09, 2024.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

