Establishment and characterization of an immortalized red river hog blood-derived macrophage cell line
- PMID: 39324137
- PMCID: PMC11422137
- DOI: 10.3389/fimmu.2024.1465952
Establishment and characterization of an immortalized red river hog blood-derived macrophage cell line
Abstract
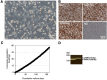
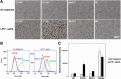
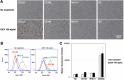
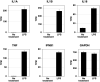
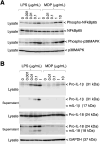
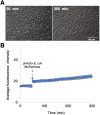
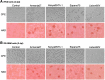
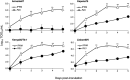
Red river hogs (RRHs) (Potamochoerus porcus), a wild species of Suidae living in Africa with a major distribution in the Guinean and Congolian forests, are natural reservoirs of African swine fever virus (ASFV) and typically are asymptomatic. Since blood and tissue macrophages of suid animals are target cell lineages of ASFV, RRH-derived macrophages are expected to play an important role in suppressing disease development in infected individuals. In the present study, we successfully isolated RRH-derived blood macrophages using co-culture techniques of RRH blood cells with porcine kidney-derived feeder cells and immortalized them by transferring SV40 large T antigen and porcine telomerase reverse transcriptase genes. The newly established macrophage cell line of the RRH-derived blood cell origin (RZJ/IBM) exhibited an Iba1-, CD172a-, and CD203a-positive typical macrophage-like phenotype and up-regulated the phosphorylation of nuclear factor-κB p65 subunit and p38 mitogen-activated protein kinase in response to the bacterial cell wall components, lipopolysaccharide (LPS) and muramyl dipeptide. In addition, RZJ/IBM cells produced the precursor form of interleukin (IL)-1β and IL-18 upon a stimulation with LPS, leading to the conversion of IL-18, but not IL-1β, into the mature form. Time-lapse live cell imaging with pHrodo dye-conjugated Escherichia coli BioParticles demonstrated the phagocytotic activity of RZJ/IBM cells. It is important to note that RZJ/IBM cells are clearly susceptible to ASFV infection and support viral replication in vitro. Therefore, the RZJ/IBM cell line provides a unique model for investigating the pathogenesis of ASFV.
Keywords: African swine fever virus; immortalization; in vitro model; macrophages; red river hog.
Copyright © 2024 Takenouchi, Masujin, Ikeda, Haraguchi, Suzuki, Uenishi, Onda and Kokuho.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Establishment and characterization of the immortalized porcine lung-derived mononuclear phagocyte cell line.Front Vet Sci. 2022 Nov 18;9:1058124. doi: 10.3389/fvets.2022.1058124. eCollection 2022. Front Vet Sci. 2022. PMID: 36467652 Free PMC article.
-
An immortalized porcine macrophage cell line competent for the isolation of African swine fever virus.Sci Rep. 2021 Feb 26;11(1):4759. doi: 10.1038/s41598-021-84237-2. Sci Rep. 2021. PMID: 33637799 Free PMC article.
-
Infection of Human Macrophage-Like Cells by African Swine Fever Virus.Front Biosci (Landmark Ed). 2024 Apr 23;29(4):164. doi: 10.31083/j.fbl2904164. Front Biosci (Landmark Ed). 2024. PMID: 38682190
-
Infection, modulation and responses of antigen-presenting cells to African swine fever viruses.Virus Res. 2018 Oct 15;258:73-80. doi: 10.1016/j.virusres.2018.10.007. Epub 2018 Oct 11. Virus Res. 2018. PMID: 30316802 Review.
-
African swine fever virus proteins involved in evading host defence systems.Vet Immunol Immunopathol. 2004 Aug;100(3-4):117-34. doi: 10.1016/j.vetimm.2004.04.002. Vet Immunol Immunopathol. 2004. PMID: 15207450 Review.
References
-
- Bordet E, Maisonnasse P, Renson P, Bouguyon E, Crisci E, Tiret M, et al. . Porcine Alveolar Macrophage-like cells are pro-inflammatory Pulmonary Intravascular Macrophages that produce large titers of Porcine Reproductive and Respiratory Syndrome Virus. Sci Rep. (2018) 8:10172. doi: 10.1038/s41598-018-28234-y - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

