T cell responses and clinical symptoms among infants with congenital cytomegalovirus infection
- PMID: 39315550
- PMCID: PMC11457853
- DOI: 10.1172/jci.insight.171029
T cell responses and clinical symptoms among infants with congenital cytomegalovirus infection
Abstract
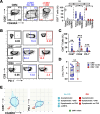
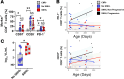
BACKGROUNDCongenital cytomegalovirus (cCMV) infection can cause developmental impairment and sensorineural hearing loss (SNHL). To determine the relationship between immune responses to cCMV infection and neurologic sequelae, T cell responses were compared for their connection to clinical symptoms at birth and neurodevelopmental outcomes.METHODSThirty cCMV-infected and 15 uninfected infants were enrolled in a single-center prospective observational case-control study. T cell pp65-specific cytokine responses; CD57, CD28, and PD-1 expression; and memory subsets were compared.RESULTSInfected neonates (73% symptomatic at birth) lacked pp65-specific cytokine-secreting T cells, with elevated frequencies of CD57+, CD28-, and PD-1+CD8+ T cells and effector memory subsets. Though frequencies overlapped between cCMV symptom groups, asymptomatic infants had higher frequencies of CD57+PD-1+CD8+ T cells. Neonates with subsequent developmental delay lacked detectable CMV-specific T cell responses, with patterns resembling those of uninfected infants. Two children with progressive SNHL had high frequencies of PD-1+CD8+ T cells over the first year compared with children without progressive SNHL.CONCLUSIONSimilar to published reports, neonatal viral antigen-specific cytokine-secreting T cell responses were not detected, but overall patterns indicate that globally differentiated memory CD8+ T cell populations were induced by cCMV infection, with higher frequencies of terminally differentiated PD-1+CD8+ T cells potentially associated with asymptomatic infection. In this cohort, a lack of in utero T cell differentiation was associated with developmental delay, and high frequencies of PD-1+CD8+ T cells persisted only in children with progressive SNHL. Further work is needed to define the specificity of these T cells and their mechanistic connection to these outcomes.FUNDINGThis study was funded through an intramural research award at Nationwide Children's Hospital, the Pediatric Infectious Disease Society Fellowship Award funded by Stanley and Susan Plotkin and Sanofi Pasteur, the Abigail Wexner Research Institute at Nationwide Children's Hospital, and the Pichichero Family Foundation Vaccines for Children Initiative Research Award from the Pediatric Infectious Diseases Society Foundation.
Keywords: Immunology; Infectious disease; Neurodevelopment; T cells.
Conflict of interest statement
Figures



Similar articles
-
Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging.PLoS One. 2014 Feb 27;9(2):e89444. doi: 10.1371/journal.pone.0089444. eCollection 2014. PLoS One. 2014. PMID: 24586783 Free PMC article.
-
T-Cell Immune Responses in Newborns and Long-Term Sequelae in Congenital Cytomegalovirus Infection (CYTRIC Study).J Pediatr. 2024 Sep;272:114084. doi: 10.1016/j.jpeds.2024.114084. Epub 2024 May 4. J Pediatr. 2024. PMID: 38705230
-
What are the neurodevelopmental outcomes of children with asymptomatic congenital cytomegalovirus infection at birth? A systematic literature review.Rev Med Virol. 2024 Jul;34(4):e2555. doi: 10.1002/rmv.2555. Rev Med Virol. 2024. PMID: 39031854 Free PMC article. Review.
-
Hearing outcomes in children with Congenital Cytomegalovirus: A multi-center, single-enterprise experience.Int J Pediatr Otorhinolaryngol. 2022 Dec;163:111376. doi: 10.1016/j.ijporl.2022.111376. Epub 2022 Nov 6. Int J Pediatr Otorhinolaryngol. 2022. PMID: 36370539
-
From diagnosis to management: current perspectives on congenital cytomegalovirus infection.Curr Opin Infect Dis. 2024 Aug 1;37(4):232-237. doi: 10.1097/QCO.0000000000001023. Epub 2024 May 14. Curr Opin Infect Dis. 2024. PMID: 38748563 Review.
References
-
- Ross SA, et al. CMV Infections. In: Elzouki AY, et al, eds. Textbook of Clinical Pediatrics. Springer; 2012:1145–1161.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

