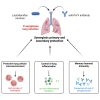
Synergy between Lactobacillus murinus and anti-PcrV antibody delivered in the airways to boost protection against Pseudomonas aeruginosa
- PMID: 39314638
- PMCID: PMC11418128
- DOI: 10.1016/j.omtm.2024.101330
Synergy between Lactobacillus murinus and anti-PcrV antibody delivered in the airways to boost protection against Pseudomonas aeruginosa
Abstract
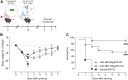
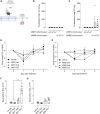
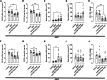
Therapeutic antibodies (Ab) have revolutionized the management of multiple illnesses including respiratory tract infections (RTIs). However, anti-infectious Ab displayed several limitations including antigen restrictiveness, narrowed therapeutic windows, and limited dose in the vicinity of the target when delivered by parenteral routes. Strategies enhancing further Ab-dependent containment of infection are currently needed. Here we showed that a combination of inhaled anti-infectious Ab and probiotics is an efficient formulation to protect against lung infection. Using a mouse model of Pseudomonas aeruginosa-induced pneumonia, we demonstrated a synergistic effect reducing both bacterial burden and pro-inflammatory response affording protection against primary and secondary infections. This is the first study showing that the local combination in the airways of anti-infective Ab and probiotics subverts suboptimal potency of Ab monotherapy and provides protection against respiratory pathogen.
Keywords: P. aeruginosa; airway delivery; pneumonia; probiotic; therapeutic antibody.
© 2024 The Author(s).
Conflict of interest statement
N.H.-V. is co-founder and scientific expert for Cynbiose Respiratory. In the past 2 years, she received consultancy fees from Eli Lilly, Argenx, and Novartis and research support from Sanofi and Aerogen Ltd. M.T. is co-founder of the start-up Carembouche.
Figures

Similar articles
-
In a murine model of acute lung infection, airway administration of a therapeutic antibody confers greater protection than parenteral administration.J Control Release. 2019 Jun 10;303:24-33. doi: 10.1016/j.jconrel.2019.04.005. Epub 2019 Apr 11. J Control Release. 2019. PMID: 30981816
-
Anti-PcrV antibody strategies against virulent Pseudomonas aeruginosa.Hum Vaccin Immunother. 2014;10(10):2843-52. doi: 10.4161/21645515.2014.971641. Hum Vaccin Immunother. 2014. PMID: 25483637 Free PMC article.
-
Mucosal administration of anti-bacterial antibodies provide long-term cross-protection against Pseudomonas aeruginosa respiratory infection.Mucosal Immunol. 2023 Jun;16(3):312-325. doi: 10.1016/j.mucimm.2023.03.005. Epub 2023 Mar 28. Mucosal Immunol. 2023. PMID: 36990281
-
Therapeutic Antibodies for the Treatment of Respiratory Tract Infections-Current Overview and Perspectives.Vaccines (Basel). 2021 Feb 13;9(2):151. doi: 10.3390/vaccines9020151. Vaccines (Basel). 2021. PMID: 33668613 Free PMC article. Review.
-
Bacteriology and treatment of infections in the upper and lower airways in patients with primary ciliary dyskinesia: adressing the paranasal sinuses.Dan Med J. 2017 May;64(5):B5361. Dan Med J. 2017. PMID: 28552099 Review.
References
-
- European Respiratory Society . European Respiratory Society; 2016. The burden of lung disease.
-
- World Health Organization . World Health Organization; 2017. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed at.
-
- World Health Organization . World Health Organization; 2020. The top 10 causes of death.
LinkOut - more resources
Full Text Sources

