Correction of osteopetrosis in the neonate oc/oc murine model after lentiviral vector gene therapy and non-genotoxic conditioning
- PMID: 39314524
- PMCID: PMC11416974
- DOI: 10.3389/fendo.2024.1450349
Correction of osteopetrosis in the neonate oc/oc murine model after lentiviral vector gene therapy and non-genotoxic conditioning
Abstract
Introduction: Autosomal recessive osteopetrosis (ARO) is a rare genetic disease, characterized by increased bone density due to defective osteoclast function. Most of the cases are due to TCIRG1 gene mutation, leading to severe bone phenotype and death in the first years of life. The standard therapy is the hematopoietic stem cell transplantation (HSCT), but its success is limited by several constraints. Conversely, gene therapy (GT) could minimize the immune-mediated complications of allogeneic HSCT and offer a prompt treatment to these patients.
Methods: The Tcirg1-defective oc/oc mouse model displays a short lifespan and high bone density, closely mirroring the human condition. In this work, we exploited the oc/oc neonate mice to optimize the critical steps for a successful therapy.
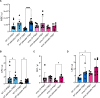
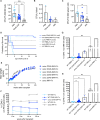
Results: First, we showed that lentiviral vector GT can revert the osteopetrotic bone phenotype, allowing long-term survival and reducing extramedullary haematopoiesis. Then, we demonstrated that plerixafor-induced mobilization can further increase the high number of HSPCs circulating in peripheral blood, facilitating the collection of adequate numbers of cells for therapeutic purposes. Finally, pre-transplant non-genotoxic conditioning allowed the stable engraftment of HSPCs, albeit at lower level than conventional total body irradiation, and led to long-term survival and correction of bone phenotype, in the absence of acute toxicity.
Conclusion: These results will pave the way to the implementation of an effective GT protocol, reducing the transplant-related complication risks in the very young and severely affected ARO patients.
Keywords: HSC mobilization; TCIRG1 gene; conditioning; gene therapy; hematopoietic stem cells; lentiviral vector; osteoclast; osteopetrosis.
Copyright © 2024 Penna, Zecchillo, Di Verniere, Fontana, Iannello, Palagano, Mantero, Cappelleri, Rizzoli, Santi, Crisafulli, Filibian, Forlino, Basso-Ricci, Scala, Scanziani, Schinke, Ficara, Sobacchi, Villa and Capo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
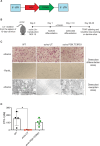
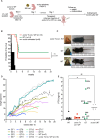
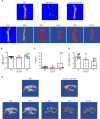

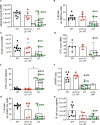
Similar articles
-
Hematopoietic Stem Cell-Targeted Neonatal Gene Therapy with a Clinically Applicable Lentiviral Vector Corrects Osteopetrosis in oc/oc Mice.Hum Gene Ther. 2019 Nov;30(11):1395-1404. doi: 10.1089/hum.2019.047. Epub 2019 Jul 3. Hum Gene Ther. 2019. PMID: 31179768
-
Fetal liver cells transplanted in utero rescue the osteopetrotic phenotype in the oc/oc mouse.Am J Pathol. 2009 Mar;174(3):727-35. doi: 10.2353/ajpath.2009.080688. Epub 2009 Feb 13. Am J Pathol. 2009. PMID: 19218349 Free PMC article.
-
Expanded circulating hematopoietic stem/progenitor cells as novel cell source for the treatment of TCIRG1 osteopetrosis.Haematologica. 2021 Jan 1;106(1):74-86. doi: 10.3324/haematol.2019.238261. Haematologica. 2021. PMID: 31949009 Free PMC article.
-
Autosomal recessive osteopetrosis: mechanisms and treatments.Dis Model Mech. 2021 May 1;14(5):dmm048940. doi: 10.1242/dmm.048940. Epub 2021 May 10. Dis Model Mech. 2021. PMID: 33970241 Free PMC article. Review.
-
Osteopetrosis: genetics, treatment and new insights into osteoclast function.Nat Rev Endocrinol. 2013 Sep;9(9):522-36. doi: 10.1038/nrendo.2013.137. Epub 2013 Jul 23. Nat Rev Endocrinol. 2013. PMID: 23877423 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

