EspH utilizes phosphoinositide and Rab binding domains to interact with plasma membrane infection sites and Rab GTPases
- PMID: 39312647
- PMCID: PMC11421376
- DOI: 10.1080/19490976.2024.2400575
EspH utilizes phosphoinositide and Rab binding domains to interact with plasma membrane infection sites and Rab GTPases
Abstract
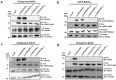
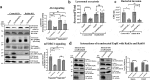
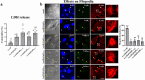
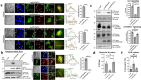
Enteropathogenic E. coli (EPEC) is a Gram-negative bacterial pathogen that causes persistent diarrhea. Upon attachment to the apical plasma membrane of the intestinal epithelium, the pathogen translocates virulence proteins called effectors into the infected cells. These effectors hijack numerous host processes for the pathogen's benefit. Therefore, studying the mechanisms underlying their action is crucial for a better understanding of the disease. We show that translocated EspH interacts with multiple host Rab GTPases. AlphaFold predictions and site-directed mutagenesis identified glutamic acid and lysine at positions 37 and 41 as Rab interacting residues in EspH. Mutating these sites abolished the ability of EspH to inhibit Akt and mTORC1 signaling, lysosomal exocytosis, and bacterial invasion. Knocking out the endogenous Rab8a gene expression highlighted the involvement of Rab8a in Akt/mTORC1 signaling and lysosomal exocytosis. A phosphoinositide binding domain with a critical tyrosine was identified in EspH. Mutating the tyrosine abolished the localization of EspH at infection sites and its capacity to interact with the Rabs. Our data suggest novel EspH-dependent mechanisms that elicit immune signaling and membrane trafficking during EPEC infection.
Keywords: Enteropathogenic e. coli; EspH; Rab GTPases; Rho GTPases; bacterial invasion; host-pathogen interactions; lysosomal exocytosis; phosphoinositide binding domain (PBD) PI3K/Akt/mTORC1 signaling; phosphoinositides (PIs); type III secreted effectors.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
EspH Suppresses Erk by Spatial Segregation from CD81 Tetraspanin Microdomains.Infect Immun. 2018 Sep 21;86(10):e00303-18. doi: 10.1128/IAI.00303-18. Print 2018 Oct. Infect Immun. 2018. PMID: 30037792 Free PMC article.
-
Enteropathogenic E. coli infection co-elicits lysosomal exocytosis and lytic host cell death.mBio. 2023 Dec 19;14(6):e0197923. doi: 10.1128/mbio.01979-23. Epub 2023 Dec 1. mBio. 2023. PMID: 38038448 Free PMC article.
-
The interplay between the Escherichia coli Rho guanine nucleotide exchange factor effectors and the mammalian RhoGEF inhibitor EspH.mBio. 2012 Jan 17;3(1):e00250-11. doi: 10.1128/mBio.00250-11. Print 2012. mBio. 2012. PMID: 22251971 Free PMC article.
-
Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements.Mol Microbiol. 2011 Jun;80(6):1420-38. doi: 10.1111/j.1365-2958.2011.07661.x. Epub 2011 May 5. Mol Microbiol. 2011. PMID: 21488979 Review.
-
Dynamics of expression, secretion and translocation of type III effectors during enteropathogenic Escherichia coli infection.Curr Opin Microbiol. 2020 Apr;54:67-76. doi: 10.1016/j.mib.2019.12.001. Epub 2020 Feb 12. Curr Opin Microbiol. 2020. PMID: 32058947 Review.
References
-
- Slater SL, Sagfors AM, Pollard DJ, Ruano-Gallego D, Frankel G. The type III secretion system of pathogenic Escherichia coli. Curr Top Microbiol Immunol. 2018;416:51–72. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
