Transferrin receptor-targeted immunostimulant for photodynamic immunotherapy against metastatic tumors through β-catenin/CREB interruption
- PMID: 39309507
- PMCID: PMC11413667
- DOI: 10.1016/j.apsb.2024.05.030
Transferrin receptor-targeted immunostimulant for photodynamic immunotherapy against metastatic tumors through β-catenin/CREB interruption
Abstract
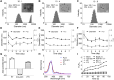
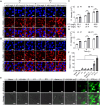
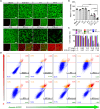
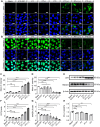
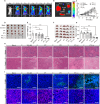
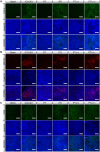
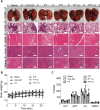
The immunosuppressive phenotype of tumor cells extensively attenuates the immune activation effects of traditional treatments. In this work, a transferrin receptor (TfR) targeted immunostimulant (PTI) is fabricated for photodynamic immunotherapy against metastatic tumors by interrupting β-catenin signal pathway. To synthesize PTI, the photosensitizer conjugated TfR targeting peptide moiety (Palmitic-K(PpIX)-HAIYPRH) is unitized to encapsulate the transcription interrupter of ICG-001. On the one hand, the recognition of PTI and TfR can promote drug delivery into tumor cells to destruct primary tumors through photodynamic therapy and initiate an immunogenic cell death with the release of tumor-associated antigens. On the other hand, PTI will interrupt the binding between β-catenin and cAMP response element-binding protein (CREB), regulating the gene transcription to downregulate programmed death ligand 1 (PD-L1) while upregulating C-C motif chemokine ligand 4 (CCL4). Furthermore, the elevated CCL4 can recruit the dendritic cells to present tumor-specific antigens and promote T cells activation and infiltration, and the downregulated PD-L1 can avoid the immune evasion of tumor cells and activate systemic anti-tumor immunity to eradicate lung metastasis. This work may inspire the development of antibody antibody-free strategy to activate systemic immune response in consideration of immunosuppressive conditions.
Keywords: C–C motif chemokine ligand 4; Immunogenic cell death; Immunotherapy; Photodynamic therapy; Programmed death ligand 1; Transferrin receptor; Tumor targeting; β-Catenin signal pathway.
© 2024 The Authors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



Similar articles
-
Chimeric peptide-engineered immunostimulant for endoplasmic reticulum targeted photodynamic immunotherapy against metastatic tumor.J Control Release. 2024 Oct;374:230-241. doi: 10.1016/j.jconrel.2024.08.013. Epub 2024 Aug 20. J Control Release. 2024. PMID: 39151829
-
Self-Assembled PD-L1 Downregulator to Boost Photodynamic Activated Tumor Immunotherapy Through CDK5 Inhibition.Small. 2024 Aug;20(33):e2311507. doi: 10.1002/smll.202311507. Epub 2024 Jun 10. Small. 2024. PMID: 38856024
-
Peptide vaccine-conjugated mesoporous carriers synergize with immunogenic cell death and PD-L1 blockade for amplified immunotherapy of metastatic spinal.J Nanobiotechnology. 2021 Aug 12;19(1):243. doi: 10.1186/s12951-021-00975-5. J Nanobiotechnology. 2021. PMID: 34384429 Free PMC article.
-
Emerging role of natural products in cancer immunotherapy.Acta Pharm Sin B. 2022 Mar;12(3):1163-1185. doi: 10.1016/j.apsb.2021.08.020. Epub 2021 Aug 21. Acta Pharm Sin B. 2022. PMID: 35530162 Free PMC article. Review.
-
Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses.Acta Pharm Sin B. 2020 May;10(5):723-733. doi: 10.1016/j.apsb.2019.09.006. Epub 2019 Sep 25. Acta Pharm Sin B. 2020. PMID: 32528824 Free PMC article. Review.
References
-
- Fan Y.B., Li Y., Yao X.D., Jin J.K., Scott A., Liu B., et al. Epithelial SOX9 drives progression and metastases of gastric adenocarcinoma by promoting immunosuppressive tumour microenvironment. Gut. 2023;72:624–637. - PubMed
-
- Cerezo-Wallis D., Contreras-Alcalde M., Troulé K., Catena X., Mucientes C., Calvo T.G., et al. Midkine rewires the melanoma microenvironment toward a tolerogenic and immune-resistant state. Nat Med. 2020;26:1865–1877. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

