Pharmacokinetics and pharmacodynamics of the factor XIa-inhibiting antibody osocimab in healthy male East Asian volunteers: Results from two phase 1 studies
- PMID: 39308062
- PMCID: PMC11417140
- DOI: 10.1002/prp2.70012
Pharmacokinetics and pharmacodynamics of the factor XIa-inhibiting antibody osocimab in healthy male East Asian volunteers: Results from two phase 1 studies
Abstract
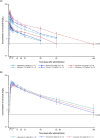
The pharmacokinetics, pharmacodynamics, immunogenicity, and safety of osocimab single doses in healthy Chinese and Japanese volunteers over 149 days were evaluated. Two phase 1 single-blinded, placebo-controlled studies with 27 Japanese and 50 Chinese participants were conducted. Osocimab was investigated with IV doses of 0.3, 1.25, and 2.5 mg/kg (Chinese study) and 0.3, 1.25, and 5.0 mg/kg (Japanese study), as well as SC doses of 3.0 and 6.0 mg/kg (Chinese study) and 6.0 mg/kg (Japanese study). The maximum plasma concentration was reached 1-3 h and 4-6 days after IV and SC administration, respectively. Osocimab exhibited a deviation from dose-proportional pharmacokinetics for AUC but not Cmax; higher doses had higher apparent clearance and disproportionately lower total exposure. A slightly lower exposure was observed in Japanese compared with Chinese volunteers after IV administration; conversely, relatively higher exposure in Japanese volunteers with SC dosing was identified. Osocimab was associated with a dose-dependent increase in activated partial thromboplastin time (aPTT). Maximal aPTT prolongations were observed 1-4 h and 2-6 days after IV and SC administration, respectively. Anti-drug antibodies of low titer were detected in 1/9 (11.1%) Japanese volunteers administered placebo and 26/40 (65.0%) Chinese volunteers administered osocimab. Adverse events were reported in 8/18 (44.4%) Japanese and 28/40 (70.0%) Chinese volunteers who received osocimab, as well as in 1/9 (11.1%) Japanese and 6/10 (60.0%) Chinese volunteers who received placebo. In conclusion, data did not suggest a clear dose-proportionality for osocimab within the investigated dose range. The effect of osocimab on aPTT was expected per its mechanism of action. Osocimab was generally well tolerated.
Keywords: cardiovascular pharmacology; pharmacokinetics; phase I.
© 2024 Bayer AG. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
Frauke Friedrichs is an employee of Bayer AG. Kensei Hashizume and Toshiaki Tanaka are employees of Bayer Yakuhin, Ltd, and may own stock in the company. Zhili Dong, Pei Liu, and Yuqin Liao are employees of Bayer Healthcare Company Ltd.
Figures
Similar articles
-
Safety, Tolerability, and Pharmacokinetics of Single Doses of Exidavnemab (BAN0805), an Anti-α-Synuclein Antibody, in Healthy Western, Caucasian, Japanese, and Han Chinese Adults.J Clin Pharmacol. 2024 Nov;64(11):1432-1442. doi: 10.1002/jcph.6103. Epub 2024 Aug 6. J Clin Pharmacol. 2024. PMID: 39105497 Clinical Trial.
-
Pharmacokinetics and Safety of Spesolimab in Healthy Chinese Subjects: An Open-Label, Phase I Study.Adv Ther. 2024 Sep;41(9):3557-3568. doi: 10.1007/s12325-024-02940-8. Epub 2024 Jul 22. Adv Ther. 2024. PMID: 39039387 Clinical Trial.
-
The Safety, Pharmacokinetics, and Pharmacodynamics of SHR2285, an Oral Small Molecule Factor XIa Inhibitor, in Healthy Chinese Volunteers.Clin Drug Investig. 2023 Jun;43(6):435-445. doi: 10.1007/s40261-023-01281-8. Epub 2023 Jun 16. Clin Drug Investig. 2023. PMID: 37326942 Clinical Trial.
-
Safety, pharmacokinetics and pharmacodynamics of BI 655064 in phase 1 clinical trials in healthy Chinese and Japanese subjects.Br J Clin Pharmacol. 2021 Apr;87(4):2000-2013. doi: 10.1111/bcp.14601. Epub 2020 Dec 9. Br J Clin Pharmacol. 2021. PMID: 33047859 Free PMC article. Clinical Trial.
-
Safety and tolerability of a humanized rabbit monoclonal antibody (SSS07) in healthy adults: Randomized double-blind placebo-controlled single ascending dose trial.Int Immunopharmacol. 2021 Feb;91:107263. doi: 10.1016/j.intimp.2020.107263. Epub 2020 Dec 28. Int Immunopharmacol. 2021. PMID: 33383447 Clinical Trial.
References
-
- Spencer FA, Ginsberg JS, Chong A, Alter DA. The relationship between unprovoked venous thromboembolism, age, and acute myocardial infarction. J Thromb Haemost. 2008;6:1507‐1513. - PubMed
-
- Piazza G, Goldhaber SZ. Venous thromboembolism and atherothrombosis: an integrated approach. Circulation. 2010;121:2146‐2150. - PubMed
-
- Winter M‐P, Schernthaner GH, Lang IM. Chronic complications of venous thromboembolism. J Thromb Haemost. 2017;15:1531‐1540. - PubMed
-
- Lee LH, Gallus A, Jindal R, Wang C, Wu C‐C. Incidence of venous thromboembolism in Asian populations: a systematic review. Thromb Haemost. 2017;117:2243‐2260. - PubMed
-
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955‐962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

