A comprehensive evaluation of dermal fibroblast therapy in clinical trials for treating skin disorders and cosmetic applications: a scoping review
- PMID: 39304949
- PMCID: PMC11416016
- DOI: 10.1186/s13287-024-03892-0
A comprehensive evaluation of dermal fibroblast therapy in clinical trials for treating skin disorders and cosmetic applications: a scoping review
Abstract
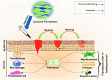
Background: Fibroblast cells have the ability to improve skin conditions through regenerative medicine and cell-based therapies. The purpose of this scoping review is to assess the contribution of fibroblast cells to skin homeostasis and extracellular matrix deposition in clinical trials involving skin disorders and cosmetic applications.
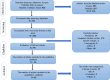
Methods: Using targeted search terms, published publications from January 2000 to August 2023 that addressed fibroblast uses in clinical trials of skin conditions were obtained from bibliographic databases like PubMed, Scopus, and Web of Science (WoS). Precise inclusion and exclusion criteria were used during the screening process. The potential benefits of induction treatment with fibroblasts lead to the choosing of clinical trials for this kind of treatment.
Results: Out of the 820 published ppapers initially identified, only 35 studies fulfilled our meticulous eligibility criteria after careful screening. To ensure clarity, we methodically eliminated any duplicate or irrelevant published papers, thereby offering a transparent account of our selection process.
Conclusion: This study highlights the advantages of fibroblast therapy in treating skin conditions such as diabetic foot, venous leg ulcers, and cosmetic reasons. Fibroblasts possess remarkable regenerating capabilities, making dermal fibroblast therapy crucial in cell-based and skin regenerative treatments. Nevertheless, additional research is required for more disorders and cosmetic applications.
Keywords: Clinical trial; Fibroblasts; Regenerative Medicine; Skin diseases; Wound healing.
© 2024. The Author(s).
Conflict of interest statement
The authors have no commercial, proprietary or financial interest in the products or companies described in this article.
Figures

Similar articles
-
Regenerative medicine in the treatment of specific dermatologic disorders: a systematic review of randomized controlled clinical trials.Stem Cell Res Ther. 2024 Jun 18;15(1):176. doi: 10.1186/s13287-024-03800-6. Stem Cell Res Ther. 2024. PMID: 38886861 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Global Trends of Exosomes Application in Clinical Trials: A Scoping Review.Stem Cell Rev Rep. 2024 Nov;20(8):2165-2193. doi: 10.1007/s12015-024-10791-7. Epub 2024 Sep 28. Stem Cell Rev Rep. 2024. PMID: 39340738 Review.
-
Bilayered bioengineered skin substitute (Apligraf): a review of its use in the treatment of venous leg ulcers and diabetic foot ulcers.BioDrugs. 2002;16(6):439-55. doi: 10.2165/00063030-200216060-00005. BioDrugs. 2002. PMID: 12463767 Review.
-
Possible benefits of food supplementation or diet in scar management: A scoping review.Scars Burn Heal. 2024 Sep 13;10:20595131241282105. doi: 10.1177/20595131241282105. eCollection 2024 Jan-Dec. Scars Burn Heal. 2024. PMID: 39280762 Free PMC article. Review.
References
-
- Ascensión AM, Fuertes-Álvarez S, Ibañez-Solé O, Izeta A, Araúzo-Bravo MJ. Human dermal fibroblast subpopulations are conserved across single-cell RNA sequencing studies. J Invest Dermatology. 2021;141:1735–e174435. 10.1016/j.jid.2020.11.028 - PubMed
-
- Zou ML, Teng YY, Wu JJ, Liu SY, Tang XY, Jia Y, Chen ZH, Zhang KW, Sun ZL, Li X, Ye JX, Xu RS, Yuan FL. Fibroblasts: Heterogeneous Cells With Potential in Regenerative Therapy for Scarless Wound Healing, Frontiers in Cell and Developmental Biology. 9 (2021) 713605. 10.3389/FCELL.2021.713605/BIBTEX - PMC - PubMed
-
- Yan WF, Murrell DF. Fibroblast-based cell therapy strategy for recessive dystrophic epidermolysis bullosa. Dermatol Clin. 2010;28:367–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

