STK40 inhibits trophoblast fusion by mediating COP1 ubiquitination to degrade P57Kip2
- PMID: 39304928
- PMCID: PMC11414097
- DOI: 10.1186/s12967-024-05360-y
STK40 inhibits trophoblast fusion by mediating COP1 ubiquitination to degrade P57Kip2
Abstract
Background: The syncytiotrophoblast (SCT) layer in the placenta serves as a crucial physical barrier separating maternal-fetal circulation, facilitating essential signal and substance exchange between the mother and fetus. Any abnormalities in its formation or function can result in various maternal syndromes, such as preeclampsia. The transition of proliferative villous cytotrophoblasts (VCT) from the mitotic cell cycle to the G0 phase is a prerequisite for VCT differentiation and their fusion into SCT. The imprinting gene P57Kip2, specifically expressed in intermediate VCT capable of fusion, plays a pivotal role in driving this key event. Moreover, aberrant expression of P57Kip2 has been linked to pathological placental conditions and adverse fetal outcomes.
Methods: Validation of STK40 interaction with P57Kip2 using rigid molecular simulation docking and co-immunoprecipitation. STK40 expression was modulated by lentivirus in BeWo cells, and the effect of STK40 on trophoblast fusion was assessed by real-time quantitative PCR, western blot, immunofluorescence, and cell viability and proliferation assays. Co-immunoprecipitation, transcriptome sequencing, and western blot were used to determine the potential mechanisms by which STK40 regulates P57Kip2.
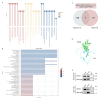
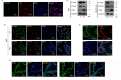
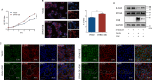
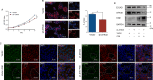
Results: In this study, STK40 has been identified as a novel interacting protein with P57Kip2, and its expression is down-regulated during the fusion process of trophoblast cells. Overexpressing STK40 inhibited cell fusion in BeWo cells while stimulating mitotic cell cycle activity. Further experiments indicated that this effect is attributed to its specific binding to the CDK-binding and the Cyclin-binding domains of P57Kip2, mediating the E3 ubiquitin ligase COP1-mediated ubiquitination and degradation of P57Kip2. Moreover, abnormally high expression of STK40 might significantly contribute to the occurrence of preeclampsia.
Conclusions: This study offers new insights into the role of STK40 in regulating the protein-level homeostasis of P57Kip2 during placental development.
Keywords: Cell fusion; P57Kip2; Placenta; Preeclampsia; STK40; Trophoblast.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Expression of p57KIP2 reduces growth and invasion, and induces syncytialization in a human placental choriocarcinoma cell line, BeWo.Placenta. 2021 Jan 15;104:168-178. doi: 10.1016/j.placenta.2020.11.010. Epub 2020 Dec 1. Placenta. 2021. PMID: 33360007
-
Appropriate expression of P57kip2 drives trophoblast fusion via cell cycle arrest.Reproduction. 2021 May 5;161(6):633-644. doi: 10.1530/REP-20-0638. Reproduction. 2021. PMID: 33812346
-
P21-activated kinase 4 regulates the cyclin-dependent kinase inhibitor p57(kip2) in human breast cancer.Anat Rec (Hoboken). 2013 Oct;296(10):1561-7. doi: 10.1002/ar.22754. Epub 2013 Jul 19. Anat Rec (Hoboken). 2013. PMID: 23873832
-
Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors.Cell Cycle. 2010 Jun 15;9(12):2342-52. doi: 10.4161/cc.9.12.11988. Epub 2010 Jun 15. Cell Cycle. 2010. PMID: 20519948 Free PMC article. Review.
-
Genetic and Epigenetic Control of CDKN1C Expression: Importance in Cell Commitment and Differentiation, Tissue Homeostasis and Human Diseases.Int J Mol Sci. 2018 Apr 2;19(4):1055. doi: 10.3390/ijms19041055. Int J Mol Sci. 2018. PMID: 29614816 Free PMC article. Review.
References
-
- Io S, Kondoh E, Chigusa Y, Kawasaki K, Mandai M, Yamada AS. New era of trophoblast research: integrating morphological and molecular approaches. Hum Reprod Update. 2020;26:611–33. - PubMed
-
- Redman CWG, Staff AC, Roberts JM. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol. 2022;226:S907–27. - PubMed
-
- Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

