Preliminary results from ASCENT-J02: a phase 1/2 study of sacituzumab govitecan in Japanese patients with advanced solid tumors
- PMID: 39302614
- PMCID: PMC11511732
- DOI: 10.1007/s10147-024-02589-x
Preliminary results from ASCENT-J02: a phase 1/2 study of sacituzumab govitecan in Japanese patients with advanced solid tumors
Abstract
Background: Sacituzumab govitecan (SG) is a Trop-2-directed antibody-drug conjugate approved outside Japan for second-line and later metastatic triple-negative breast cancer (mTNBC), based on the ASCENT study (NCT02574455). We report SG safety and efficacy in an open-label, phase 1/2 bridging study in Japanese patients with advanced solid tumors (ASCENT-J02; NCT05101096; jRCT2031210346).
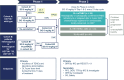
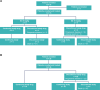
Methods: Phase 1 was a standard 3 + 3 design. Patients received intravenous SG 6 mg/kg, escalating to 10 mg/kg, on Days 1 and 8 per 21-day cycle; primary endpoints were safety, incidence of dose-limiting toxicity/toxicities (DLTs), and determination of the recommended phase 2 dose (RP2D). In the multicohort phase 2 study, patients in the mTNBC cohort with previously treated disease received SG at the RP2D; primary endpoint was independent review committee (IRC)-assessed objective response rate (ORR; RECIST v1.1). Safety was a secondary endpoint.
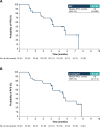
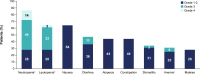
Results: In phase 1 (N = 15), one DLT (grade 3 elevated transaminases) occurred with SG 10 mg/kg; RP2D was SG 10 mg/kg regardless of UGT1A1 status. In phase 2, 36 patients with mTNBC received SG 10 mg/kg. At median follow-up of 6.1 months, IRC-assessed ORR was 25.0% (95% CI 12.1-42.2; P = 0.0077). Median progression-free survival was 5.6 months (95% CI 3.9-not reached [NR]); median overall survival was NR. No treatment-emergent adverse events led to discontinuation or death.
Conclusions: SG RP2D was established as 10 mg/kg in Japanese patients. SG showed efficacy in Japanese patients with previously treated mTNBC, a manageable safety profile, and no new safety signals, consistent with the previous ASCENT study.
Keywords: Antibody–drug conjugate; Japanese patients; Metastatic triple-negative breast cancer; Phase 2; Sacituzumab govitecan.
© 2024. The Author(s).
Conflict of interest statement
YN reports research funding from Gilead Sciences, Inc; and honoraria from AstraZeneca, Eisai, Ono, Guardant, Takeda, Eli Lilly, Novartis, Pfizer, Chugai, PDR Pharma, Nihon Kayaku, Taiho, Bristol, Bayer, and Daiichi Sankyo. SN, TI, TN, and TK report nothing to disclose. NKS reports research funding from Gilead Sciences, Inc; and honoraria from Chugai, Daiichi Sankyo, Kyowa Kirin, Nippon Kayaku, Pfizer, Taiho, Eisai, AstraZeneca, and Zena. YY reports honoraria from AstraZeneca, Chugai, Kyowa-Kirin, Novartis, Lilly, Pfizer, Daiichi Sankyo, Nippon-Kayaku, Taiho, Eisai, Takeda, MSD, Sysmex and Exact Science; and other financial or non-financial interests with the Japanese Breast Cancer Society and the Japan Breast Cancer Research Group. NM reports honoraria from AstraZeneca, Chugai, Daiichi Sankyo, Eli Lilly, and Pfizer; and uncompensated leadership roles for JBCRG, Japanese Breast Cancer Society (JBCS), and Japan Society of Clinical Oncology (JSCO). KM reports research funding from Gilead Sciences, Inc; and honoraria from MSD, Kyowa-Kirin, Eli Lilly, Daiichi-Sankyo, Eisai, and Chugai. KS reports research funding from Amge
Figures
Similar articles
-
Real-world use patterns, effectiveness, and tolerability of sacituzumab govitecan for second-line and later-line treatment of metastatic triple-negative breast cancer in the United States.Breast Cancer Res Treat. 2024 Nov;208(1):203-214. doi: 10.1007/s10549-024-07412-9. Epub 2024 Jun 21. Breast Cancer Res Treat. 2024. PMID: 38904892 Free PMC article.
-
Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer.N Engl J Med. 2019 Feb 21;380(8):741-751. doi: 10.1056/NEJMoa1814213. N Engl J Med. 2019. PMID: 30786188 Clinical Trial.
-
Sacituzumab govitecan in metastatic triple-negative breast cancer patients treated at Institut Curie Hospitals: efficacy, safety, and impact of brain metastases.Breast Cancer. 2024 Jul;31(4):572-580. doi: 10.1007/s12282-024-01565-7. Epub 2024 Apr 10. Breast Cancer. 2024. PMID: 38600429 Free PMC article.
-
Sacituzumab govitecan: past, present and future of a new antibody-drug conjugate and future horizon.Future Oncol. 2022 Sep;18(28):3199-3215. doi: 10.2217/fon-2022-0407. Epub 2022 Sep 7. Future Oncol. 2022. PMID: 36069628 Review.
-
Role of sacituzumab govitecan in solid tumors.J Oncol Pharm Pract. 2022 Oct;28(7):1617-1623. doi: 10.1177/10781552221092359. Epub 2022 Apr 11. J Oncol Pharm Pract. 2022. PMID: 35404158 Review.
References
-
- Allison KH, Hammond MEH, Dowsett M et al (2020) Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP Guideline Update. J Clin Oncol 38(12):1346–1366 - PubMed
-
- Wolff AC, Somerfield MR, Dowsett M et al (2023) Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists Guideline Update. J Clin Oncol 41(22):3867–3872 - PubMed
-
- Foundation for Promotion of Cancer Research (2023) Cancer Statistics in Japan - 2023. Available at: https://ganjoho.jp/public/qa_links/report/statistics/pdf/cancer_statisti.... Accessed 19 Oct 2023
-
- Iwase H, Kurebayashi J, Tsuda H et al (2010) Clinicopathological analyses of triple negative breast cancer using surveillance data from the Registration Committee of the Japanese Breast Cancer Society. Breast Cancer 17(2):118–124 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

