Advances in the cell biology of the trafficking and processing of amyloid precursor protein: impact of familial Alzheimer's disease mutations
- PMID: 39302110
- PMCID: PMC11555708
- DOI: 10.1042/BCJ20240056
Advances in the cell biology of the trafficking and processing of amyloid precursor protein: impact of familial Alzheimer's disease mutations
Abstract
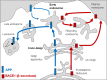
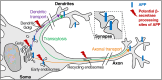
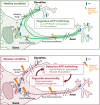
The production of neurotoxic amyloid-β peptides (Aβ) is central to the initiation and progression of Alzheimer's disease (AD) and involves sequential cleavage of the amyloid precursor protein (APP) by β- and γ-secretases. APP and the secretases are transmembrane proteins and their co-localisation in the same membrane-bound sub-compartment is necessary for APP cleavage. The intracellular trafficking of APP and the β-secretase, BACE1, is critical in regulating APP processing and Aβ production and has been studied in several cellular systems. Here, we summarise the intracellular distribution and transport of APP and its secretases, and the intracellular location for APP cleavage in non-polarised cells and neuronal models. In addition, we review recent advances on the potential impact of familial AD mutations on APP trafficking and processing. This is critical information in understanding the molecular mechanisms of AD progression and in supporting the development of novel strategies for clinical treatment.
Keywords: Alzheimers disease; Golgi apparatus; amyloid precursor protein; endosomal sorting; neurons; trafficking.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
The role of membrane trafficking in the processing of amyloid precursor protein and production of amyloid peptides in Alzheimer's disease.Biochim Biophys Acta Biomembr. 2019 Apr 1;1861(4):697-712. doi: 10.1016/j.bbamem.2018.11.013. Epub 2019 Jan 11. Biochim Biophys Acta Biomembr. 2019. PMID: 30639513 Review.
-
Spatial-Temporal Mapping Reveals the Golgi as the Major Processing Site for the Pathogenic Swedish APP Mutation: Familial APP Mutant Shifts the Major APP Processing Site.Traffic. 2024 Mar;25(3):e12932. doi: 10.1111/tra.12932. Traffic. 2024. PMID: 38528836
-
Alternative Selection of β-Site APP-Cleaving Enzyme 1 (BACE1) Cleavage Sites in Amyloid β-Protein Precursor (APP) Harboring Protective and Pathogenic Mutations within the Aβ Sequence.J Biol Chem. 2016 Nov 11;291(46):24041-24053. doi: 10.1074/jbc.M116.744722. Epub 2016 Sep 29. J Biol Chem. 2016. PMID: 27687728 Free PMC article.
-
Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis.Eur J Neurosci. 2013 Jun;37(12):1962-9. doi: 10.1111/ejn.12235. Eur J Neurosci. 2013. PMID: 23773065
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

