Type V collagen-induced nasal tolerance prevents lung damage in an experimental model: new evidence of autoimmunity to collagen V in COPD
- PMID: 39301030
- PMCID: PMC11410637
- DOI: 10.3389/fimmu.2024.1444622
Type V collagen-induced nasal tolerance prevents lung damage in an experimental model: new evidence of autoimmunity to collagen V in COPD
Abstract
Background: Chronic obstructive pulmonary disease (COPD) has been linked to immune responses to lung-associated self-antigens. Exposure to cigarette smoke (CS), the main cause of COPD, causes chronic lung inflammation, resulting in pulmonary matrix (ECM) damage. This tissue breakdown exposes collagen V (Col V), an antigen typically hidden from the immune system, which could trigger an autoimmune response. Col V autoimmunity has been linked to several lung diseases, and the induction of immune tolerance can mitigate some of these diseases. Evidence suggests that autoimmunity to Col V might also occur in COPD; thus, immunotolerance to Col V could be a novel therapeutic approach.
Objective: The role of autoimmunity against collagen V in COPD development was investigated by analyzing the effects of Col V-induced tolerance on the inflammatory response and lung remodeling in a murine model of CS-induced COPD.
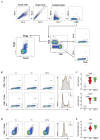
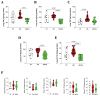
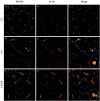
Methods: Male C57BL/6 mice were divided into three groups: one exposed to CS for four weeks, one previously tolerated for Col V and exposed to CS for four weeks, and one kept in clean air for the same period. Then, we proceeded with lung functional and structural evaluation, assessing inflammatory cells in bronchoalveolar lavage fluid (BALF) and inflammatory markers in the lung parenchyma, inflammatory cytokines in lung and spleen homogenates, and T-cell phenotyping in the spleen.
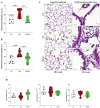
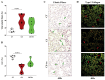
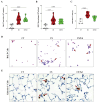
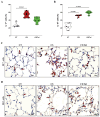
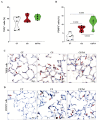
Results: CS exposure altered the structure of elastic and collagen fibers and increased the pro-inflammatory immune response, indicating the presence of COPD. Col V tolerance inhibited the onset of emphysema and prevented structural changes in lung ECM fibers by promoting an immunosuppressive microenvironment in the lung and inducing Treg cell differentiation.
Conclusion: Induction of nasal tolerance to Col V can prevent inflammatory responses and lung remodeling in experimental COPD, suggesting that autoimmunity to Col V plays a role in COPD development.
Keywords: animal models; autoimmunity; chronic obstructive pulmonary disease; cigarette smoking; collagen type V; immune tolerance; pulmonary emphysema; regulatory T cell.
Copyright © 2024 Robertoni, Velosa, Oliveira, Almeida, Silveira, Queiroz, Lobo, Contini, Baldavira, Carrasco, Fernezlian, Sato, Capelozzi, Lopes and Teodoro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A shift in the collagen V antigenic epitope leads to T helper phenotype switch and immune response to self-antigen leading to chronic lung allograft rejection.Clin Exp Immunol. 2012 Jan;167(1):158-68. doi: 10.1111/j.1365-2249.2011.04486.x. Clin Exp Immunol. 2012. PMID: 22132895 Free PMC article.
-
Proposition of a novel animal model of systemic sclerosis induced by type V collagen in C57BL/6 mice that reproduces fibrosis, vasculopathy and autoimmunity.Arthritis Res Ther. 2019 Dec 11;21(1):278. doi: 10.1186/s13075-019-2052-2. Arthritis Res Ther. 2019. PMID: 31829272 Free PMC article.
-
Guggulsterone protects against cigarette smoke-induced COPD linked lung inflammation.Cell Biochem Biophys. 2024 Jun;82(2):1145-1158. doi: 10.1007/s12013-024-01265-1. Epub 2024 Apr 12. Cell Biochem Biophys. 2024. PMID: 38609738
-
Vitamin D deficiency exacerbates COPD-like characteristics in the lungs of cigarette smoke-exposed mice.Respir Res. 2015 Sep 16;16(1):110. doi: 10.1186/s12931-015-0271-x. Respir Res. 2015. PMID: 26376849 Free PMC article.
-
Role of Regulatory T Cells in Disturbed Immune Homeostasis in Patients With Chronic Obstructive Pulmonary Disease.Front Immunol. 2020 Apr 28;11:723. doi: 10.3389/fimmu.2020.00723. eCollection 2020. Front Immunol. 2020. PMID: 32411140 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

