Preclinical Development of SHR-1819, a Potent Humanized IL-4Rα Antibody for Treating Type 2 Inflammatory Diseases
- PMID: 39296644
- PMCID: PMC11410029
- DOI: 10.2147/JIR.S471963
Preclinical Development of SHR-1819, a Potent Humanized IL-4Rα Antibody for Treating Type 2 Inflammatory Diseases
Abstract
Background: Interleukin (IL)-4 and IL-13 are critical pathogenic factors for type 2 inflammation-related allergic diseases, sharing the mutual receptor subunit IL-4Rα. However, it was ineffective for certain type 2 inflammation diseases by targeting IL-4, IL-13 ligand alone or both in clinical studies. The work presented herein aimed to evaluate the preclinical efficacy and pharmacokinetics profile of a novel monoclonal antibody against IL-4Rα, SHR-1819, as a promising therapy for type 2 inflammation diseases.
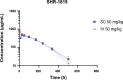
Methods: SHR-1819 was generated through immunization by C57BL/6 mice with recombinant hIL-4Rα protein, followed by humanization and affinity maturation. Then, its binding properties with IL-4Rα were determined using surface plasmon resonance (SPR) and ELISA. In vitro inhibitory effects of SHR-1819 were assessed on hIL-4-/hIL-13-induced cell proliferation and signal transducer and activator of transcription 6 (STAT6) signaling activation. In vivo efficacy of SHR-1819 was evaluated in several type 2 inflammatory diseases models, including asthma, atopic dermatitis (AD), and allergic rhinitis (AR) by using hIL-4/hIL-4Rα transgenic mice. Furthermore, the pharmacokinetic (PK) profiles of SHR-1819 were characterized.
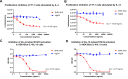
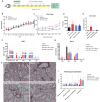
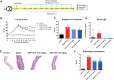
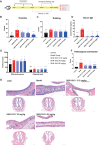
Results: SHR-1819 showed high binding affinity to human IL-4Rα and effectively blocked IL-4Rα at sub-nanomolar concentration. In vitro assays indicated that SHR-1819 significantly inhibited TF-1 cell proliferation and STAT6 activation induced by hIL-4/hIL-13. In the asthma model, SHR-1819 could reduce airway hyperresponsiveness, decrease serum IgE levels, and alleviated inflammatory lung cell infiltration. In the AD model, SHR-1819 could significantly alleviate inflammatory and skin symptoms. In the AR model, it could remarkably decrease the frequencies of nasal rubbing and sneezing, and inflammatory cell infiltration in nasal tissues. These in vivo efficacy studies demonstrated the therapeutic potential of SHR-1819 in preclinical disease models. Moreover, subcutaneous administration of SHR-1819 exhibited favorable bioavailability in mice.
Conclusion: The results supported SHR-1819 as a promising preclinical candidate for the treatment of type 2 inflammatory diseases, including asthma, AD and AR.
Keywords: IL-13; IL-4; IL-4Rα; type 2 inflammatory diseases.
© 2024 Zhao et al.
Conflict of interest statement
The authors declare no competing interest in this work.
Figures

Similar articles
-
Increased miR-124-3p alleviates type 2 inflammatory response in allergic rhinitis via IL-4Rα.Inflamm Res. 2022 Nov;71(10-11):1271-1282. doi: 10.1007/s00011-022-01614-x. Epub 2022 Aug 3. Inflamm Res. 2022. PMID: 35922673 Free PMC article.
-
A novel inhalable nanobody targeting IL-4Rα for the treatment of asthma.J Allergy Clin Immunol. 2024 Oct;154(4):1008-1021. doi: 10.1016/j.jaci.2024.05.027. Epub 2024 Jun 11. J Allergy Clin Immunol. 2024. PMID: 38871183
-
Murine IL-4 is able to signal via chimeric human IL-4Ralpha/mouse gamma-chain receptor.Mol Immunol. 2008 Mar;45(5):1327-36. doi: 10.1016/j.molimm.2007.09.009. Epub 2007 Oct 29. Mol Immunol. 2008. PMID: 18029018
-
Chronic rhinosinusitis with nasal polyps: mechanistic insights from targeting IL-4 and IL-13 via IL-4Rα inhibition with dupilumab.Expert Rev Clin Immunol. 2020 Dec;16(12):1115-1125. doi: 10.1080/1744666X.2021.1847083. Epub 2020 Nov 15. Expert Rev Clin Immunol. 2020. PMID: 33148074 Review.
-
Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma.Curr Mol Med. 2008 Aug;8(5):384-92. doi: 10.2174/156652408785161032. Curr Mol Med. 2008. PMID: 18691065 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

