Impact of Hepatitis B Virus Point-of-care DNA Viral Load Testing Compared With Laboratory-based Standard-of-care Approaches on Uptake of HBV Viral Load Testing, Treatment, and Turnaround Times: A Systematic Review and Meta-analysis
- PMID: 39296343
- PMCID: PMC11409893
- DOI: 10.1093/ofid/ofae483
Impact of Hepatitis B Virus Point-of-care DNA Viral Load Testing Compared With Laboratory-based Standard-of-care Approaches on Uptake of HBV Viral Load Testing, Treatment, and Turnaround Times: A Systematic Review and Meta-analysis
Abstract
Background: Point-of-care (PoC) hepatitis B virus (HBV) DNA viral load (VL) assays represent an alternative to laboratory-based standard-of-care (SoC) VL assays to accelerate diagnosis and treatment. We evaluated the impact of using PoC versus SoC approaches on the uptake of VL testing, treatment, and turnaround times from testing to treatment across the HBV care cascade.
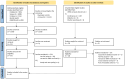
Methods: We searched 5 databases, 6 conference websites, and contacted manufacturers for unpublished reports, for articles with or without a comparator (SoC VL testing), and had data on the uptake of VL testing, treatment, or turnaround times between hepatitis B surface antigen (HBsAg) testing, VL testing, and treatment in the cascade. We performed a random-effects meta-analysis on rates of VL testing and treatment initiation.
Results: Six studies, composing 9 arms, were included. Three PoC arms reported less than 1 day between screening for HBsAg positivity and VL testing, and the other one (2 arms) reported it between 7 and 11 days. Five arms reported the time to available VL test results (<1 day). Three studies reported 1-8 days between VL testing results and treatment initiation. Two studies reported the turnaround times between a positive HBsAg screening and treatment initiation (the same day and 27 days). Overall, 84.1% of those with HBsAg positivity were tested for DNA VL and 88.3% of eligible people initiated treatment.
Conclusions: HBV PoC DNA testing appears to be associated with a turnaround time of <1 day for receipt of VL results and appears associated with high rates of DNA testing and initiation of treatment among those eligible.
Clinical trials registration: PROSPERO CRD42023398440.
Keywords: hepatitis B virus; meta-analysis; point-of-care; systematic review; viral load.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: no reported conflicts.
Figures
Similar articles
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article.
-
Interventions for supporting pregnant women's decision-making about mode of birth after a caesarean.Cochrane Database Syst Rev. 2013 Jul 30;2013(7):CD010041. doi: 10.1002/14651858.CD010041.pub2. Cochrane Database Syst Rev. 2013. PMID: 23897547 Free PMC article. Review.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Aug 27;8:CD007807. doi: 10.1002/14651858.CD007807.pub4. PMID: 28752910 Free PMC article. Updated. Review.
-
A multicomponent psychosocial intervention to reduce substance use by adolescents involved in the criminal justice system: the RISKIT-CJS RCT.Public Health Res (Southampt). 2023 Mar;11(3):1-77. doi: 10.3310/FKPY6814. Public Health Res (Southampt). 2023. PMID: 37254608
References
-
- Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet 2018; 392:2313–24. - PubMed
-
- Jeng WJ, Papatheodoridis GV, Lok ASF. Hepatitis B. Lancet 2023; 401:1039–52. - PubMed
-
- Hsu YC, Huang DQ, Nguyen MH. Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat Rev Gastroenterol Hepatol 2023; 20(8):524–37. - PubMed
-
- WHO. Hepatitis B . 2021. Available at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Accessed 24 June 2022.
Grants and funding
LinkOut - more resources
Full Text Sources

