Rationally modified SNX-class Hsp90 inhibitors disrupt extracellular fibronectin assembly without intracellular Hsp90 activity
- PMID: 39290382
- PMCID: PMC11403943
- DOI: 10.1039/d4md00501e
Rationally modified SNX-class Hsp90 inhibitors disrupt extracellular fibronectin assembly without intracellular Hsp90 activity
Abstract
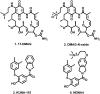
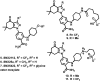
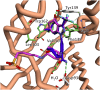
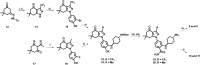
Despite Hsp90's well documented promise as a target for developing cancer chemotherapeutics, its inhibitors have struggled to progress through clinical trials. This is, in part, attributed to the cytoprotective compensatory heat shock response (HSR) stimulated through intracellular Hsp90 inhibition. Beyond its intracellular role, secreted extracellular Hsp90 (eHsp90) interacts with numerous pro-oncogenic extracellular clients. This includes fibronectin, which in the tumour microenvironment enhances cell invasiveness and metastasis. Through the rational modification of known Hsp90 inhibitors (SNX2112 and SNX25a) we developed four Hsp90 inhibitory compounds, whose alterations restricted their interaction with intracellular Hsp90 and did not stimulate the HSR. Two of the modified cohort (compounds 10 and 11) were able to disrupt the assembly of the extracellular fibronectin network at non-cytotoxic concentrations, and thus represent promising new tool compounds for studying the druggability of eHsp90 as a target for inhibition of tumour invasiveness and metastasis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Extracellular Hsp90 Binds to and Aligns Collagen-1 to Enhance Breast Cancer Cell Invasiveness.Cancers (Basel). 2023 Oct 31;15(21):5237. doi: 10.3390/cancers15215237. Cancers (Basel). 2023. PMID: 37958410 Free PMC article.
-
SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase-dependent cancers.Clin Cancer Res. 2008 Jan 1;14(1):240-8. doi: 10.1158/1078-0432.CCR-07-1667. Clin Cancer Res. 2008. PMID: 18172276 Free PMC article.
-
Extracellular Hsp90 (eHsp90) as the actual target in clinical trials: intentionally or unintentionally.Int Rev Cell Mol Biol. 2013;303:203-35. doi: 10.1016/B978-0-12-407697-6.00005-2. Int Rev Cell Mol Biol. 2013. PMID: 23445811 Free PMC article. Review.
-
Methods to Assess the Impact of Hsp90 Chaperone Function on Extracellular Client MMP2 Activity.Methods Mol Biol. 2023;2693:221-232. doi: 10.1007/978-1-0716-3342-7_17. Methods Mol Biol. 2023. PMID: 37540438 Free PMC article.
-
Extracellular Heat Shock Protein-90 (eHsp90): Everything You Need to Know.Biomolecules. 2022 Jun 29;12(7):911. doi: 10.3390/biom12070911. Biomolecules. 2022. PMID: 35883467 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

