Fusobacterium nucleatum induces invasive growth and angiogenic responses in malignant oral keratinocytes that are cell line- and bacterial strain-specific
- PMID: 39286811
- PMCID: PMC11402903
- DOI: 10.3389/fcimb.2024.1417946
Fusobacterium nucleatum induces invasive growth and angiogenic responses in malignant oral keratinocytes that are cell line- and bacterial strain-specific
Abstract
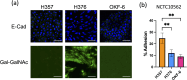
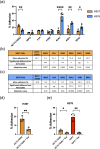
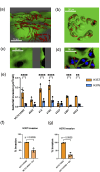
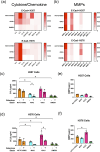
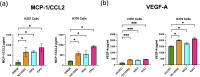
Fusobacterium nucleatum is an anaerobic commensal of the oral cavity recently reported to be associated with cancers of the gastrointestinal tract and oral squamous cell carcinoma (OSCC). In this study, we investigate the impact on oral keratinocytes of infection with a genetically diverse set of strains of F. nucleatum subsp. polymorphum recovered from patients with oral dysplasia (n=6). We employed H357 oral keratinocytes derived from a stage 1 OSCC and H376 cells derived from a stage 3 OSCC. Adhesion phenotypes were strain specific, with 3/6 clinical isolates examined exhibiting higher adherence to the stage 3 H376 cell line. Conversely, intracellular invasion was greatest in the H357 cells and was associated with specific transcriptional responses including autophagy and keratinization. Infection of both H357 and H376 cell lines induced transcriptional and cytokine responses linked to cancer cell migration and angiogenesis. F. nucleatum infection induced greater levels of MMP9 secretion in the H376 cell line which was associated with enhanced motility and invasion phenotypes. Additionally, the degree of F. nucleatum induced invasive growth by H376 cells varied between different clinical isolates of F. nucleatum subsp. polymorphum. Blockage of CCL5 signalling using the inhibitor metCCL5 resulted in reduced keratinocyte invasion. F. nucleatum infection also induced expression of the pro-angiogenic chemokine MCP-1 and the angiogenic growth factor VEGF-A resulting in increased capillary-like tube formation in HUVEC cells, most significantly in H376 cells. Treatment of HUVEC cells with resveratrol, a VEGF-A signalling inhibitor, significantly attenuated F. nucleatum induced tube formation. Our data indicate that the outcomes of F. nucleatum-oral cell interactions can vary greatly depending on the bacterial genotype and the malignant phenotype of the host cell.
Keywords: Fusobacterium nucleatum; angiogenesis; inflammation; invasion; oral cancer.
Copyright © 2024 Selvaraj, McManus, Healy and Moran.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Conversion from epithelial to partial-EMT phenotype by Fusobacterium nucleatum infection promotes invasion of oral cancer cells.Sci Rep. 2021 Jul 22;11(1):14943. doi: 10.1038/s41598-021-94384-1. Sci Rep. 2021. PMID: 34294795 Free PMC article.
-
About a Possible Impact of Endodontic Infections by Fusobacterium nucleatum or Porphyromonas gingivalis on Oral Carcinogenesis: A Literature Overview.Int J Mol Sci. 2024 May 7;25(10):5083. doi: 10.3390/ijms25105083. Int J Mol Sci. 2024. PMID: 38791123 Free PMC article. Review.
-
Epithelial-mesenchymal transition in oral cancer cells induced by prolonged and persistent Fusobacterium nucleatum stimulation.J Oral Biosci. 2024 Sep;66(3):594-604. doi: 10.1016/j.job.2024.05.006. Epub 2024 May 21. J Oral Biosci. 2024. PMID: 38782256
-
Fusobacterium nucleatum promotes epithelial-mesenchymal transiton through regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 signaling pathway.FEBS J. 2020 Sep;287(18):4032-4047. doi: 10.1111/febs.15233. Epub 2020 Feb 12. FEBS J. 2020. PMID: 31997506 Free PMC article.
-
Fusobacterium nucleatum and oral cancer: a critical review.BMC Cancer. 2021 Nov 13;21(1):1212. doi: 10.1186/s12885-021-08903-4. BMC Cancer. 2021. PMID: 34774023 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

