Tff1-expressing Tregs in lung prevent exacerbation of Bleomycin-induced pulmonary fibrosis
- PMID: 39286257
- PMCID: PMC11402662
- DOI: 10.3389/fimmu.2024.1440918
Tff1-expressing Tregs in lung prevent exacerbation of Bleomycin-induced pulmonary fibrosis
Abstract
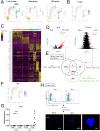
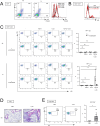
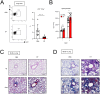
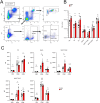
Bleomycin (BLM) induces lung injury, leading to inflammation and pulmonary fibrosis. Regulatory T cells (Tregs) maintain self-tolerance and control host immune responses. However, little is known about their involvement in the pathology of pulmonary fibrosis. Here we show that a unique Treg subset expressing trefoil factor family 1 (Tff1) emerges in the BLM-injured lung. These Tff1-expressing Tregs (Tff1-Tregs) were induced by IL-33. Moreover, although Tff1 ablation in Tregs did not change the pathological condition, selective ablation of Tff1-Tregs using an intersectional genetic method promoted pro-inflammatory features of macrophages in the injured lung and exacerbated the fibrosis. Taken together, our study revealed the presence of a unique Treg subset expressing Tff1 in BLM-injured lungs and their critical role in the injured lung to ameliorate fibrosis.
Keywords: Bleomycin; Tff1; Treg; VeDTR; fibrosis.
Copyright © 2024 Okamoto, Kuratani, Okuzaki, Kamiyama, Kobayashi, Sasai and Yamamoto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Resolution of bleomycin-induced murine pulmonary fibrosis via a splenic lymphocyte subpopulation.Respir Res. 2018 Apr 24;19(1):71. doi: 10.1186/s12931-018-0783-2. Respir Res. 2018. PMID: 29690905 Free PMC article.
-
Fasting alleviates bleomycin-induced lung inflammation and fibrosis via decreased Tregs and monocytes.Adv Med Sci. 2024 Sep;69(2):303-311. doi: 10.1016/j.advms.2024.07.004. Epub 2024 Jul 8. Adv Med Sci. 2024. PMID: 38986767
-
Aryl hydrocarbon receptor signals attenuate lung fibrosis in the bleomycin-induced mouse model for pulmonary fibrosis through increase of regulatory T cells.Arthritis Res Ther. 2020 Feb 7;22(1):20. doi: 10.1186/s13075-020-2112-7. Arthritis Res Ther. 2020. PMID: 32033616 Free PMC article.
-
Deregulated immune cell recruitment orchestrated by c-MET impairs pulmonary inflammation and fibrosis.Respir Res. 2024 Jun 22;25(1):257. doi: 10.1186/s12931-024-02884-1. Respir Res. 2024. PMID: 38909206 Free PMC article.
-
Allies or enemies? The effect of regulatory T cells and related T lymphocytes on the profibrotic environment in bleomycin-injured lung mouse models.Clin Exp Med. 2023 Aug;23(4):1075-1088. doi: 10.1007/s10238-022-00945-7. Epub 2022 Nov 20. Clin Exp Med. 2023. PMID: 36403186 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

