This is a preprint.
Calpains Orchestrate Secretion of Annexin-containing Microvesicles during Membrane Repair
- PMID: 39282443
- PMCID: PMC11398502
- DOI: 10.1101/2024.09.05.611512
Calpains Orchestrate Secretion of Annexin-containing Microvesicles during Membrane Repair
Abstract
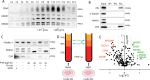
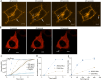
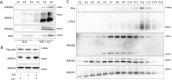
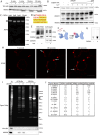
Microvesicles (MVs) are membrane-enclosed, plasma membrane-derived particles released by cells from all branches of life. MVs have utility as disease biomarkers and may participate in intercellular communication; however, physiological processes that induce their secretion are not known. Here, we isolate and characterize annexin-containing MVs and show that these vesicles are secreted in response to the calcium influx caused by membrane damage. The annexins in these vesicles are cleaved by calpains. After plasma membrane injury, cytoplasmic calcium-bound annexins are rapidly recruited to the plasma membrane and form a scab-like structure at the lesion. In a second phase, recruited annexins are cleaved by calpains-1/2, disabling membrane scabbing. Cleavage promotes annexin secretion within MVs. Our data supports a new model of plasma membrane repair, where calpains relax annexin-membrane aggregates in the lesion repair scab, allowing secretion of damaged membrane and annexins as MVs. We anticipate that cells experiencing plasma membrane damage, including muscle and metastatic cancer cells, secrete these MVs at elevated levels.
Figures

Similar articles
-
Annexin A6 mediates calcium-dependent exosome secretion during plasma membrane repair.Elife. 2023 May 19;12:e86556. doi: 10.7554/eLife.86556. Elife. 2023. PMID: 37204294 Free PMC article.
-
Annexins A2, A6 and Fetuin-A Affect the Process of Mineralization in Vesicles Derived from Human Osteoblastic hFOB 1.19 and Osteosarcoma Saos-2 Cells.Int J Mol Sci. 2021 Apr 13;22(8):3993. doi: 10.3390/ijms22083993. Int J Mol Sci. 2021. PMID: 33924370 Free PMC article.
-
Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization.Circ Res. 2011 Jun 24;109(1):e1-12. doi: 10.1161/CIRCRESAHA.110.238808. Epub 2011 May 12. Circ Res. 2011. PMID: 21566214
-
Interdisciplinary Synergy to Reveal Mechanisms of Annexin-Mediated Plasma Membrane Shaping and Repair.Cells. 2020 Apr 21;9(4):1029. doi: 10.3390/cells9041029. Cells. 2020. PMID: 32326222 Free PMC article. Review.
-
Annexins are instrumental for efficient plasma membrane repair in cancer cells.Semin Cell Dev Biol. 2015 Sep;45:32-8. doi: 10.1016/j.semcdb.2015.10.028. Epub 2015 Oct 20. Semin Cell Dev Biol. 2015. PMID: 26498035 Review.
References
-
- Koerdt S. N., Gerke V., Annexin A2 is involved in Ca2+-dependent plasma membrane repair in primary human endothelial cells. Biochim. Biophys. Acta Mol. Cell Res. 1864, 1046–1053 (2017). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
