Mucosal immunity in upper and lower respiratory tract to MERS-CoV
- PMID: 39281686
- PMCID: PMC11392799
- DOI: 10.3389/fimmu.2024.1358885
Mucosal immunity in upper and lower respiratory tract to MERS-CoV
Abstract
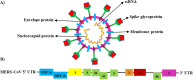
Introduction: Middle East respiratory syndrome coronavirus (MERS-CoV) has emerged as a deadly pathogen with a mortality rate of up to 36.2%. MERS-CoV can cause severe respiratory tract disease and multiorgan failure. Therefore, therapeutic vaccines are urgently needed. This intensive review explores the human immune responses and their immunological mechanisms during MERS-CoV infection in the mucosa of the upper and lower respiratory tracts (URT and LRT, respectively).
Objective: The aim of this study is to provide a valuable, informative, and critical summary of the protective immune mechanisms against MERS-CoV infection in the URT/LRT for the purpose of preventing and controlling MERS-CoV disease and designing effective therapeutic vaccines.
Methods: In this review, we focus on the immune potential of the respiratory tract following MERS-CoV infection. We searched PubMed, Embase, Web of Science, Cochrane, Scopus, and Google Scholar using the following terms: "MERS-CoV", "B cells", "T cells", "cytokines", "chemokines", "cytotoxic", and "upper and lower respiratory tracts".
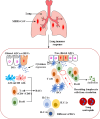
Results: We found and included 152 studies in this review. We report that the cellular innate immune response, including macrophages, dendritic cells, and natural killer cells, produces antiviral substances such as interferons and interleukins to prevent the virus from spreading. In the adaptive and humoral immune responses, CD4+ helper T cells, CD8+ cytotoxic T cells, B cells, and plasma cells protect against MERS-CoV infection in URT and LRT.
Conclusion: The human nasopharynx-associated lymphoid tissue (NALT) and bronchus-associated lymphoid tissue (BALT) could successfully limit the spread of several respiratory pathogens. However, in the case of MERS-CoV infection, limited research has been conducted in humans with regard to immunopathogenesis and mucosal immune responses due to the lack of relevant tissues. A better understanding of the immune mechanisms of the URT and LRT is vital for the design and development of effective MERS-CoV vaccines.
Keywords: MERS-CoV; chemokines; cytokines; immune cells; lung; mucosal; tonsils; upper and lower respiratory tracts.
Copyright © 2024 Shrwani, Mahallawi, Mohana, Algaissi, Dhayhi, Sharwani, Gadour, Aldossari, Asiri, Kameli, Asiri, Asiri, Sherwani, Cunliffe and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures.Lancet Respir Med. 2020 Jul;8(7):687-695. doi: 10.1016/S2213-2600(20)30193-4. Epub 2020 May 7. Lancet Respir Med. 2020. PMID: 32386571 Free PMC article.
-
The influence of interferon-lambda on restricting Middle East Respiratory Syndrome Coronavirus replication in the respiratory epithelium.Antiviral Res. 2020 Aug;180:104860. doi: 10.1016/j.antiviral.2020.104860. Epub 2020 Jun 19. Antiviral Res. 2020. PMID: 32565134 Free PMC article.
-
MERS-CoV infection is associated with downregulation of genes encoding Th1 and Th2 cytokines/chemokines and elevated inflammatory innate immune response in the lower respiratory tract.Cytokine. 2020 Feb;126:154895. doi: 10.1016/j.cyto.2019.154895. Epub 2019 Nov 6. Cytokine. 2020. PMID: 31706200 Free PMC article.
-
Modulation of the immune response by Middle East respiratory syndrome coronavirus.J Cell Physiol. 2019 Mar;234(3):2143-2151. doi: 10.1002/jcp.27155. Epub 2018 Aug 26. J Cell Physiol. 2019. PMID: 30146782 Free PMC article. Review.
-
Middle East Respiratory Syndrome Coronavirus (MERS-CoV): Infection, Immunological Response, and Vaccine Development.J Immunol Res. 2019 Apr 7;2019:6491738. doi: 10.1155/2019/6491738. eCollection 2019. J Immunol Res. 2019. PMID: 31089478 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

