In vivo CAR T cell therapy against angioimmunoblastic T cell lymphoma
- PMID: 39272178
- PMCID: PMC11401350
- DOI: 10.1186/s13046-024-03179-5
In vivo CAR T cell therapy against angioimmunoblastic T cell lymphoma
Abstract
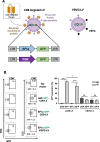
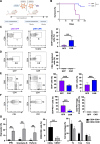
Background: For angioimmunoblastic T cell lymphoma (AITL), a rare cancer, no specific treatments are available and survival outcome is poor. We previously developed a murine model for AITL that mimics closely human disease and allows to evaluate new treatments. As in human AITL, the murine CD4+ follicular helper T (Tfh) cells are drivers of the malignancy. Therefore, chimeric antigen receptor (CAR) T cell therapy might represent a new therapeutic option.
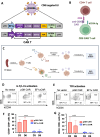
Methods: To prevent fratricide among CAR T cells when delivering an CD4-specific CAR, we used a lentiviral vector (LV) encoding an anti-CD4 CAR, allowing exclusive entry into CD8 T cells.
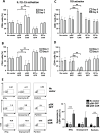
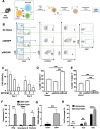
Results: These anti-CD4CAR CD8-targeted LVs achieved in murine AITL biopsies high CAR-expression levels in CD8 T cells. Malignant CD4 Tfh cells were eliminated from the mAITL lymphoma, while the CAR + CD8 T cells expanded upon encounter with the CD4 receptor and were shaped into functional cytotoxic cells. Finally, in vivo injection of the CAR + CD8-LVs into our preclinical AITL mouse model carrying lymphomas, significantly prolonged mice survival. Moreover, the in vivo generated functional CAR + CD8 T cells efficiently reduced neoplastic T cell numbers in the mAITL tumors.
Conclusion: This is the first description of in vivo generated CAR T cells for therapy of a T cell lymphoma. The strategy described offers a new therapeutic concept for patients suffering from CD4-driven T cell lymphomas.
Keywords: AITL; CAR T; CD8-targeted virus envelope; Cancer therapy; In vivo gene therapy; Lentiviral vector; Preclinical model; Pseudotyping; T cell lymphoma.
© 2024. The Author(s).
Conflict of interest statement
E.V. and C.J.B are listed as inventors on patents on receptor-targeted LVs that have been licensed out. All other authors declare no competing interests.
Figures
Similar articles
-
Inhibition of choline metabolism in an angioimmunoblastic T-cell lymphoma preclinical model reveals a new metabolic vulnerability as possible target for treatment.J Exp Clin Cancer Res. 2024 Feb 6;43(1):43. doi: 10.1186/s13046-024-02952-w. J Exp Clin Cancer Res. 2024. PMID: 38321568 Free PMC article.
-
In Vivo Generation of CAR T Cells Selectively in Human CD4+ Lymphocytes.Mol Ther. 2020 Aug 5;28(8):1783-1794. doi: 10.1016/j.ymthe.2020.05.005. Epub 2020 May 16. Mol Ther. 2020. PMID: 32485137 Free PMC article.
-
Angioimmunoblastic T-cell lymphoma contains multiple clonal T-cell populations derived from a common TET2 mutant progenitor cell.J Pathol. 2020 Mar;250(3):346-357. doi: 10.1002/path.5376. Epub 2020 Jan 16. J Pathol. 2020. PMID: 31859368 Free PMC article.
-
[Diagnosis and treatment of angioimmunoblastic T-cell lymphoma and related cancers].Rinsho Ketsueki. 2019;60(9):1221-1228. doi: 10.11406/rinketsu.60.1221. Rinsho Ketsueki. 2019. PMID: 31597847 Review. Japanese.
-
[Molecular mechanisms of angioimmunoblastic T-cell lymphoma].Rinsho Ketsueki. 2015 Mar;56(3):246-52. doi: 10.11406/rinketsu.56.246. Rinsho Ketsueki. 2015. PMID: 25876776 Review. Japanese.
References
-
- Lachenal F, Berger F, Ghesquières H, Biron P, Hot A, Callet-Bauchu E, Chassagne C, Coiffier B, Durieu I, Rousset H, et al. Angioimmunoblastic T-cell lymphoma: clinical and laboratory features at diagnosis in 77 patients. Medicine (Baltimore). 2007;86:282–92. 10.1097/MD.0b013e3181573059. 10.1097/MD.0b013e3181573059 - DOI - PubMed
-
- de Leval L, Parrens M, Le Bras F, Jais J-P, Fataccioli V, Martin A, Lamant L, Delarue R, Berger F, Arbion F, et al. Angioimmunoblastic T-cell lymphoma is the most common T-cell lymphoma in two distinct French information data sets. Haematologica. 2015;100:e361-364. 10.3324/haematol.2015.126300. 10.3324/haematol.2015.126300 - DOI - PMC - PubMed
-
- Mourad N, Mounier N, Brière J, Raffoux E, Delmer A, Feller A, Meijer CJLM, Emile J-F, Bouabdallah R, Bosly A, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463–70. 10.1182/blood-2007-08-105759. 10.1182/blood-2007-08-105759 - DOI - PMC - PubMed
-
- Alizadeh AA, Advani RH. Evaluation and management of angioimmunoblastic T-cell lymphoma: a review of current approaches and future strategies. Clin Adv Hematol Oncol. 2008;6:899–909. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

