AnophelesModel: An R package to interface mosquito bionomics, human exposure and intervention effects with models of malaria intervention impact
- PMID: 39269993
- PMCID: PMC11424000
- DOI: 10.1371/journal.pcbi.1011609
AnophelesModel: An R package to interface mosquito bionomics, human exposure and intervention effects with models of malaria intervention impact
Abstract
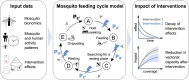
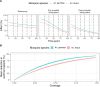
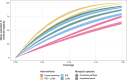
In recent decades, field and semi-field studies of malaria transmission have gathered geographic-specific information about mosquito ecology, behaviour and their sensitivity to interventions. Mathematical models of malaria transmission can incorporate such data to infer the likely impact of vector control interventions and hence guide malaria control strategies in various geographies. To facilitate this process and make model predictions of intervention impact available for different geographical regions, we developed AnophelesModel. AnophelesModel is an online, open-access R package that quantifies the impact of vector control interventions depending on mosquito species and location-specific characteristics. In addition, it includes a previously published, comprehensive, curated database of field entomological data from over 50 Anopheles species, field data on mosquito and human behaviour, and estimates of vector control effectiveness. Using the input data, the package parameterizes a discrete-time, state transition model of the mosquito oviposition cycle and infers species-specific impacts of various interventions on vectorial capacity. In addition, it offers formatted outputs ready to use in downstream analyses and by other models of malaria transmission for accurate representation of the vector-specific components. Using AnophelesModel, we show how the key implications for intervention impact change for various vectors and locations. The package facilitates quantitative comparisons of likely intervention impacts in different geographical settings varying in vector compositions, and can thus guide towards more robust and efficient malaria control recommendations. The AnophelesModel R package is available under a GPL-3.0 license at https://github.com/SwissTPH/AnophelesModel.
Copyright: © 2024 Golumbeanu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Bionomics of malaria vectors in Lao PDR, 2018-2020: entomological surveillance as a key tool for malaria elimination.Malar J. 2023 Oct 21;22(1):319. doi: 10.1186/s12936-023-04754-5. Malar J. 2023. PMID: 37865735 Free PMC article.
-
Attacking the mosquito on multiple fronts: Insights from the Vector Control Optimization Model (VCOM) for malaria elimination.PLoS One. 2017 Dec 1;12(12):e0187680. doi: 10.1371/journal.pone.0187680. eCollection 2017. PLoS One. 2017. PMID: 29194440 Free PMC article.
-
Bionomics and ecology of Anopheles merus along the East and Southern Africa coast.Parasit Vectors. 2021 Jan 28;14(1):84. doi: 10.1186/s13071-021-04582-z. Parasit Vectors. 2021. PMID: 33509262 Free PMC article. Review.
-
Predicting the impact of outdoor vector control interventions on malaria transmission intensity from semi-field studies.Parasit Vectors. 2021 Jan 20;14(1):64. doi: 10.1186/s13071-020-04560-x. Parasit Vectors. 2021. PMID: 33472661 Free PMC article.
-
Malaria entomological profile in Tanzania from 1950 to 2010: a review of mosquito distribution, vectorial capacity and insecticide resistance.Tanzan J Health Res. 2011 Dec;13(5 Suppl 1):319-31. Tanzan J Health Res. 2011. PMID: 26591987 Review.
References
-
- World Health Organization. World malaria report 2022. 2022 [Available from: https://apps.who.int/iris/rest/bitstreams/1484818/retrieve.
-
- Sinka ME. Global distribution of the dominant vector species of malaria. Anopheles mosquitoes-New insights into malaria vectors: IntechOpen; 2013.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

